Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries
Abstract
:1. Introduction
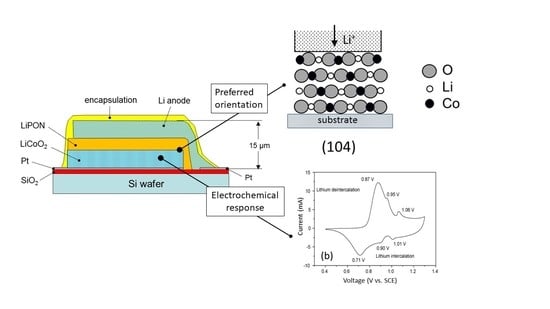
2. All Solid-State Lithium Microbatteries
3. Growth of LCO Thin Films
3.1. RF-Magnetron Sputtering
3.2. Electron Cyclotron Resonance (ECR) Sputtering
4. Influence of Preparation Conditions
4.1. Influence of the Substrate
4.2. Deposition Conditions
5. Electrochemical Properties of Sputtered LCO Films
5.1. Charge–Discharge Behavior
5.2. Li+ Ion Diffusion
5.3. Solid-Electrolyte/Electrode Interface
5.4. Effect of Doping
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Balkanski, M.; Julien, C.; Emery, J.Y. Integrable lithium solid-state microbatteries. J. Power Sources 1989, 26, 615–622. [Google Scholar] [CrossRef]
- Bates, J.B.; Gruzalski, G.R.; Dudney, N.J.; Luck, C.F.; Yu, X.H.; Jones, S.D. Rechargeable thin-film lithium microbatteries. Solid State Technol. 1993, 36, 59–64. [Google Scholar]
- Bates, J.B.; Dudney, N.J.; Gruzalski, G.R.; Zuhr, R.A.; Choudhury, A.; Luck, C.F.; Robertson, J.D. Fabrication and characterization of amorphous lithium electrolyte thin films and rechargeable thin-film batteries. J. Power Sources 1993, 43, 103–110. [Google Scholar] [CrossRef]
- Bates, J.B.; Gruzalski, G.R.; Dudney, N.J.; Luck, C.F.; Yu, X.H. Rechargeable thin-film lithium microbatteries. Solid State Ion. 1994, 70–71, 619–628. [Google Scholar] [CrossRef]
- Twentyman, J. Thin Film Batteries Set for Solid (State) Growth, Web Ref. (2017). Available online: https://internetofbusiness.com/thin-film-batteries-set-solid-state-growth (accessed on 3 November 2017).
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x < 1): A new cathode material for batteries of high energy density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A. Solid-state redox reactions of LiCoO2 (R-3m) for 4-volt secondary lithium cells. J. Electrochem. Soc. 1994, 141, 2972–2977. [Google Scholar] [CrossRef]
- Yebka, B.; Julien, C. Lithium intercalation in sputtered MoO3 films. Ionics 1997, 3, 83–88. [Google Scholar] [CrossRef]
- Julien, C. Solid State Batteries. In Handbook of Solid-State Electrochemistry; Gellings, P.J., Bouwmeester, H.J.M., Eds.; CRC Press: Roca Raton, FL, USA, 1997; Chapter 11; pp. 371–406. [Google Scholar]
- Jayanth-Babu, K.; Jeevan-Kumar, P.; Hussain, O.M.; Julien, C.M. Influence of annealing temperature on microstructural and electrochemical properties of rf-sputtered LiMn2O4 film cathodes. J. Solid State Electrochem. 2012, 16, 3383–3390. [Google Scholar] [CrossRef]
- Washizu, E.; Yamamoto, A.; Abe, Y.; Kawamura, M.; Sasaki, K. Optical and electrochromic properties of RF reactively sputtered WO3 films. Solid State Ion. 2003, 165, 175–180. [Google Scholar] [CrossRef]
- Mazas-Brandariz, D.; Senaris-Rodriguez, M.A.; Castro-Garcia, S.; Camacho-Lopez, M.A.; Julien, C. Structural properties of LiNi1-yCoyO2 (0 ≤ y ≤ 1) synthesized by wet chemistry via malic-acid assisted technique. Ionics 1999, 5, 345–350. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Nagayama, M.; Iwakoshi, Y.; Komori, H. Comparative study of LiCoO2, LiNi1/2Co1/2O2 and LiNiO2 for 4 volt secondary lithium cells. Electrochim. Acta 1993, 38, 1159–1167. [Google Scholar] [CrossRef]
- Stockhoff, T.; Gallasch, T.; Berkemeier, F.; Schmitz, G. Ion beam sputter-deposition of LiCoO2 films. Thin Solid Film. 2012, 520, 3668–3674. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Influence of the PVdF binder on the stability of LiCoO2 electrodes. Electrochem. Commun. 2005, 7, 1298–1304. [Google Scholar] [CrossRef]
- Ruffo, R.; Wessells, C.; Huggins, R.A.; Cui, Y. Electrochemical behavior of LiCoO2 as aqueous lithium-ion battery electrodes. Electrochem. Commun. 2009, 11, 247–249. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Levasseur, S.; Weill, F.; Delmas, C. Probing lithium vacancy ordering in O3 layered LixCoO2 (x≈0.5): An electron diffraction study. J. Electrochem. Soc. 2003, 150, A366–A373. [Google Scholar] [CrossRef]
- Wang, G.J.; Qu, Q.T.; Wang, B.; Shi, Y.; Tian, S.; Wu, Y.P.; Holze, R. Electrochemical behavior of LiCoO2 in a saturated aqueous Li2SO4 solution. Electrochim. Acta 2009, 54, 1199–1203. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Pulsed-laser deposited films for microbatteries. Coatings 2019, 9, 386. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.; Ueda, A.; Evans, C.D. Thin-film lithium and lithium ion batteries. Solid State Ion. 2000, 135, 33–45. [Google Scholar] [CrossRef]
- Hayashi, M.; Takahashi, M.; Sakurai, Y. Preparation of positive LiCoO2 films by electron cyclotron resonance (ECR) plasma sputtering method and its application to all-solid-state thin-film lithium batteries. J. Power Sources 2007, 174, 990–995. [Google Scholar] [CrossRef]
- Takahashi, M.; Hayashi, M.; Shodai, T. Characterization of all-solid-state secondary batteries with LiCoO2 thin films prepared by ECR sputtering as positive electrodes. J. Power Sources 2009, 189, 191–196. [Google Scholar] [CrossRef]
- Navone, C.; Tintignac, S.; Pereira-Ramos, J.P.; Baddour-Hadjean, R.; Salot, R. Electrochemical behaviour of sputtered c-V2O5 and LiCoO2 thin films for solid state lithium microbatteries. Solid State Ion. 2011, 192, 343–346. [Google Scholar] [CrossRef]
- Tintignac, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.P.; Salot, R. High rate bias sputtered LiCoO2 thin films as positive electrode for all-solid-state lithium microbatteries. Electrochim. Acta 2014, 146, 472–476. [Google Scholar] [CrossRef]
- Kim, H.-S.; Oh, Y.; Kang, K.H.; Kim, J.H.; Kim, J.; Yoon, C.S. Characterization of sputter-deposited LiCoO2 thin film grown on NASICON-type electrolyte for application in all-solid-state rechargeable lithium battery. Acs Appl. Mater. Interfaces 2017, 9, 16063–16070. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-W.; Choi, H.; Park, H.Y.; Park, G.B.; Lee, K.C.; Lee, H.-J. High rate induced structural changes in thin-film lithium batteries on flexible substrate. J. Power Sources 2010, 195, 8275–8279. [Google Scholar] [CrossRef]
- Song, S.-W.; Lee, K.-C.; Park, H.-Y. High-performance flexible all-solid-state microbatteries based on solid electrolyte of lithium boron oxynitride. J. Power Sources 2016, 328, 311–317. [Google Scholar] [CrossRef]
- Xiao, D.-L.; Tong, J.; Feng, Y.; Zhong, G.-H.; Li, W.-J.; Yang, C.-L. Improved performance of all-solid-state lithium batteries using LiPON electrolyte prepared with Li-rich sputtering target. Solid State Ion. 2018, 324, 202–206. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Lee, S.H.; Cho, S.B.; Nam, S.C. Influence of two-step heat treatment on sputtered lithium cobalt oxide thin films. J. Electrochem. Soc. 2011, 158, A1313–A1319. [Google Scholar] [CrossRef]
- Yu, X.; Bates, J.B.; Jellison, G.E., Jr.; Hart, F.X. A stable thin-film electrolyte: Lithium phosphorus oxynitride. J. Electrochem. Soc. 1997, 144, 524–532. [Google Scholar] [CrossRef]
- Xia, H.; Wang, H.L.; Xiao, W.; Lai, M.O.; Lu, L. Thin film Li electrolytes for all-solid-state micro-batteries. Int. J. Surface Sci. Eng. 2009, 3, 23–43. [Google Scholar] [CrossRef]
- Bates, J.B.; Dudney, N.J.; Gruzalski, G.R.; Zuhr, R.A.; Choudhury, A.; Luck, C.F. Electrical properties of amorphous lithium electrolyte thin films. Solid State Ion. 1992, 53–56, 647–654. [Google Scholar]
- Dudney, N.J. Solid-state thin-film rechargeable batteries. Mater. Sci. Eng. B 2005, 116, 245–249. [Google Scholar] [CrossRef]
- Choi, C.H.; Cho, W.I.; Cho, B.W.; Kim, H.S.; Yoon, Y.S.; Tak, Y.S. Radio-frequency magnetron sputtering power effect on the ionic conductivities of LiPON films. Electrochem. Solid-State Lett. 2002, 5, A14–A17. [Google Scholar]
- Hamon, Y.; Douard, A.; Sabary, F.; Marcel, C.; Vinatier, P.; Pecquenard, B.; Levasseur, A. Influence of sputtering conditions on ionic conductivity of LiPON thin films. Solid State Ion. 2006, 177, 257–261. [Google Scholar] [CrossRef]
- Le Van-Jodin, L.; Claudel, A.; Secouard, C.; Sabary, F.; Barnes, J.P.; Martin, S. Role of the chemical composition and structure on the electrical properties of a solid state electrolyte: Case of a highly conductive LiPON. Electrochim. Acta 2018, 259, 742–751. [Google Scholar] [CrossRef]
- Fleutot, B.; Pecquenard, B.; Martinez, H.; Letellier, M.; Levasseur, A. Investigation of the local structure of LiPON thin films to better understand the role of nitrogen on their performance. Solid State Ion. 2011, 186, 29–36. [Google Scholar] [CrossRef]
- Wang, B.; Bates, J.B.; Hart, F.X.; Sales, B.C.; Zuhr, R.A.; Robertson, J.D. Characterization of thin-film rechargeable lithium batteries with lithium cobalt oxide cathodes. J. Electrochem. Soc. 1996, 143, 3203–3213. [Google Scholar] [CrossRef]
- Fragnaud, P.; Brousse, T.; Schleich, D.M. Characterization of sprayed and sputter deposited LiCoO2 thin films for rechargeable microbatteries. J. Power Sources 1996, 63, 187–191. [Google Scholar] [CrossRef]
- Julien, C.; Camacho-Lopez, M.A.; Escobar-Alarcon, L.; Haro-Poniatowski, E. Fabrication of LiCoO2 thin-film cathodes for rechargeable lithium microbatteries. Mater. Chem. Phys. 2001, 68, 210–216. [Google Scholar] [CrossRef]
- Zhang, H.; Demaray, R.E. Deposition of LiCoO2. U.S. Patent 8636876B2, 28 January 2014. [Google Scholar]
- Kutbee, A.T.; Ghoneim, M.T.; Ahmad, S.M.; Hussain, M.M. Free-form flexible lithium-ion microbattery. IEEE Trans. Nanotechnol. 2016, 15, 402–408. [Google Scholar] [CrossRef]
- Kutbee, A.T.; Bahabry, R.R.; Alamoudi, K.O.; Ghoneim, M.T.; Cordero, M.D.; Almuslem, A.S.; Gumus, A.; Diallo, E.M.; Nassar, J.M.; Hussain, A.M.; et al. Flexible and bio-compatible high-performance solid-state micro-battery for implantable orthodontic system. NPJ Flexible Electron. 2017, 1, 7. [Google Scholar]
- Nam, S.C.; Park, H.Y.; Lim, Y.C.; Choi, K.G.; Lee, K.C.; Park, G.B.; Kim, J.B. Reserve battery having all solid state thin film battery. U.S. Patent 8389144, 5 March 2013. [Google Scholar]
- Bates, J.B.; Dudney, N.J.; Neudecker, B.J.; Hart, F.X.; Jun, H.P.; Hackney, S.A. Preferred orientation of polycrystalline LiCoO2 films. J. Electrochem. Soc. 2000, 147, 59–70. [Google Scholar] [CrossRef]
- Trask, J.; Anapolsky, A.; Cardozo, B.; Januar, E.; Kumar, L.; Miller, M.; Brown, R.; Bhardwaj, R. Optimization of 10-µm, sputtered, LiCoO2 cathodes to enable higher energy density solid state batteries. J. Power Sources 2017, 350, 56–64. [Google Scholar] [CrossRef]
- Yoon, Y.; Park, C.; Kim, J.; Shin, D. Lattice orientation control of lithium cobalt oxide cathode film for all-solid-state thin film batteries. J. Power Sources 2013, 226, 186–190. [Google Scholar] [CrossRef]
- Wei, G.; Haas, T.E.; Goldner, R.B. Thin films of lithium cobalt oxide. Solid State Ion. 1992, 58, 115–122. [Google Scholar] [CrossRef]
- Dudney, N.J.; Jang, Y.I. Analysis of thin-film lithium batteries with cathodes of 50 nm to 4 µm thick LiCoO2. J. Power Sources 2003, 119–121, 300–304. [Google Scholar]
- Whitacre, J.F.; West, W.C.; Ratnakumar, B.V. The influence of target history and deposition geometry on RF magnetron sputtered LiCoO2 thin films. J. Power Sources 2001, 103, 134–139. [Google Scholar] [CrossRef]
- Huang, L.; Mank, R.M.; Chen, Y. Method to improve LiCoO2 morphology in thin film batteries. U.S. Patent 9899661B2, 20 February 2018. [Google Scholar]
- Hart, F.X.; Bates, J.B. Lattice model calculation of the strain energy density and other properties of crystalline LiCoO2. J. Appl. Phys. 1998, 83, 7560–7566. [Google Scholar] [CrossRef]
- Jeevan-Kumar, P.; Jayanth-Babu, K.; Hussain, O.M. Characteristics of HT-LiCoO2 cathode films synthesized by rf magnetron sputtering. AIP Conf. Proc. 2012, 1447, 779–780. [Google Scholar]
- Ribeiro, J.F.; Sousa, R.; Silva, M.F.; Goncalves, L.M.; Silva, M.M.; Correia, J.H. Thin-film materials for solid-state rechargeable lithium batteries. Ecs Trans. 2013, 45, 139–142. [Google Scholar] [CrossRef]
- Hayashi, M.; Takahashi, M.; Shodai, T. Preparation and electrochemical properties of pure lithium cobalt films by electron cyclotron resonance sputtering. J. Power Sources 2009, 189, 416–422. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Buckingham, S.; Johnson, L.G. Thin film battery. U.S. Patent 2004/0018424 A1, 29 January 2004. [Google Scholar]
- Liao, C.L.; Lee, Y.H.; Fung, K.Z. The films growth and electrochemical properties of rf-sputtered LiCoO2 thin films. J. Alloy. Compd 2007, 436, 303–308. [Google Scholar] [CrossRef]
- Ziebert, C.; Ketterer, B.; Rinke, M.; Adelhelm, C.; Ulrich, S.; Zum Gahr, K.-H.S.; Indris, S.; Schimmel, T. Constitution, microstructure, and battery performance of magnetron sputtered Li–Co–O thin film cathodes for lithium-ion batteries as a function of the working gas pressure. Surf. Coatings Technol. 2010, 205, 1589–1594. [Google Scholar]
- Hukan-Yudar, H.; Pat, S.; Ozen, S.; Mohammadigharehbagh, R.; Musaoglu, C.; Korkmaz, S.; Pat, Z. Microstructural, surface and electrochemical properties of the nano layered LiCoO2 thin film cathode for Li ion battery. Vacuum 2018, 152, 248–251. [Google Scholar] [CrossRef]
- Kusuril, Y.R.; Penki, T.; Nookala, M.; Morgen, P.; Gowravaram, M.R. Investigations on sputter deposited LiCoO2 thin films from powder target. Adv. Mater. Lett. 2013, 4, 615–620. [Google Scholar]
- Joo, H.; Lee, H.; Cho, G.; Nam, T.; Huh, S.; Choi, B.; Jueong, H.; Noh, J. Influence of the metal-induced crystallization on the structural and electrochemical properties of sputtered LiCoO2 thin films. Thin Solid Film. 2017, 641, 53–58. [Google Scholar] [CrossRef]
- Liao, C.-L.; Fung, K.-Z. Lithium cobalt oxide cathode film prepared by rf sputtering. J. Power Sources 2004, 128, 263–269. [Google Scholar] [CrossRef]
- Kim, W.S. Characteristics of LiCoO2 thin film cathodes according to the annealing ambient for the post-annealing process. J. Power Sources 2004, 134, 103–109. [Google Scholar] [CrossRef]
- Noh, J.; Cho, G.; Jung, K.; Kang, W.; Ha, C.; Ahn, H.; Ahn, J.; Nam, T.; Kim, K. Fabrication of LiCoO2 thin film cathodes by DC magnetron sputtering method. Mater. Res. Bull. 2012, 47, 2823–2826. [Google Scholar] [CrossRef]
- Jung, K.-T.; Cho, G.-B.; Kim, K.-W.; Nam, T.-H.; Jeong, H.-M.; Huh, S.-C.; Chung, H.-S.; Noh, J.-P. Influence of the substrate texture on the structural and electrochemical properties of sputtered LiCoO2 thin films. Thin Solid Film. 2013, 546, 414–417. [Google Scholar] [CrossRef]
- Xie, J.; Imanishi, N.; Hirano, A.; Matsumura, T.A.; Takeda, Y.; Yamamoto, O. Kinetics investigation of a preferential (104) plane oriented LiCoO2 thin film prepared by RF magnetron sputtering. Solid State Ion. 2007, 178, 1218–1224. [Google Scholar]
- Pan, H.; Yang, Y. Effects of radio-frequency sputtering powers on the micro-structures and electrochemical properties of LiCoO2 thin film electrodes. J. Power Sources 2009, 189, 633–637. [Google Scholar] [CrossRef]
- Bouwman, P.J.; Boukamp, B.A.; Bouwmeester, H.J.M.; Wondergem, H.J.; Notten, P.H.L. Structural analysis of submicrometer LiCoO2 films. J. Electrochem. Soc. 2001, 148, A311–A317. [Google Scholar] [CrossRef]
- Liao, C.L.; Wu, M.T.; Yen, J.H.; Leu, I.C.; Fung, K.Z. Preparation of RF-sputtered lithium cobalt oxide nanorods by using porous anodic alumina (PAA) template. J. Alloys Compd. 2006, 414, 302–309. [Google Scholar] [CrossRef]
- Cho, J.; Losego, M.D.; Zhang, H.G.; Kim, H.; Zuo, J.; Petrov, I.; Cahill, D.G.; Braun, P.V. Electrochemically tunable thermal conductivity of lithium cobalt oxide. Nat. Commun. 2014, 5, 4035. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Lee, S.J.; Baik, H.K.; Lee, H.Y.; Jang, S.K.; Lee, S.M. Substrate effect on the microstructure and electrochemical properties in the deposition of a thin film LiCoO2 electrode. Electrochem. Solid State Lett. 1999, 2, 512–515. [Google Scholar]
- Jeevan-Kumar, P.; Jayanth-Babu, K.; Hussain, O.M.; Julien, C.M. RF-sputtered LiCoO2 thick films: Microstructure and electrochemical performance as cathodes in aqueous and non-aqueous microbatteries. Ionics 2013, 19, 421–428. [Google Scholar] [CrossRef]
- Jeong, E.; Hong, C.; Tak, Y.; Nam, S.C.; Cho, S. Investigation of interfacial resistance between LiCoO2 cathode and LiPON electrolyte in the thin film battery. J. Power Sources 2006, 159, 223–226. [Google Scholar] [CrossRef]
- Jeevan-Kumar, P.; Jayanth-Babu, K.; Hussain, O.M. Enhanced electrochemical properties of as grown LiCoO2 film cathodes: Influence of silicon substrate surface texturing. Mater. Chem. Phys. 2014, 143, 536–544. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, Z.; Du, G.; Zhang, P.; Liu, H.K. LiCoO2 cathode thin film fabricated by RF sputtering for lithium ion microbatteries. Surf. Coatings Technol. 2010, 204, 1710–1714. [Google Scholar] [CrossRef]
- Vieira, E.M.F.; Ribeiro, J.F.; Sousa, R.; Silva, M.M.; Dupont, L.; Gonçalves, L.M. Titanium oxide adhesion layer for high temperature annealed Si/Si3N4/TiOx/Pt/LiCoO2 battery structures. J. Electron. Mater. 2016, 45, 910–916. [Google Scholar] [CrossRef]
- Hu, Q.; Li, R.; Zhang, X.; Gao, Q.; Wang, M.; Shi, H.; Xiao, Z.; Chu, P.K.; Huang, A. Lithium ion trapping mechanism of SiO2 in LiCoO2 based memristors. Sci. Rep. 2019, 9, 5081. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Imanishi, N.; Matsumura, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Orientation dependence of Li–ion diffusion kinetics in LiCoO2 thin films prepared by RF magnetron sputtering. Solid State Ion. 2008, 179, 362–370. [Google Scholar] [CrossRef]
- Jeon, S.W.; Lim, J.K.; Lim, S.H.; Lee, S.M. As-deposited LiCoO2 thin film cathodes prepared by rf magnetron sputtering. Electrochim. Acta 2005, 51, 268–273. [Google Scholar] [CrossRef]
- Pracharova, J.; Pridal, J.; Bludska, J.; Jakubec, I.; Vorlicek, V.; Malkova, Z.; Makris, T.D.; Giorgi, R.; Jastrabik, L. LiCoO2 thin-film cathodes grown by RF sputtering. J. Power Sources 2002, 108, 204–212. [Google Scholar] [CrossRef]
- Rao, K.J.; Benqlilou-Moudden, H.; Couturier, G.; Vinatier, P.; Levasseur, A. Structure and electrical properties of sputtered lithium cobaltite thin films. Mater. Res. Bull. 2002, 37, 1353–1367. [Google Scholar] [CrossRef]
- Benqlilou-Moudden, H.; Blondiaux, G.; Vinatier, P.; Levasseur, A. Amorphous lithium cobalt and nickel oxides thin films: Preparation and characterization by RBS and PIGE. Thin Solid Film. 1998, 333, 16–19. [Google Scholar] [CrossRef]
- Park, Y.; Nam, S.C.; Lim, Y.C.; Choi, K.G.; Lee, K.C.; Park, G.B.; Park, H.; Cho, S.B. Influence of sputtering gas pressure on the LiCoO2 thin film cathode post-annealed at 400 °C. Korean J. Chem. Eng. 2006, 23, 832–837. [Google Scholar]
- Nimisha, C.S.; Mohan-Rao, G. Simulation and experimental study on compositional evolution of Li-Co in LiCoO2 thin films during sputter deposition. J. Appl. Phys. 2011, 109, 114910. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, S.R.; Lee, Y.J.; Cho, B.W.; Cho, W.I. Bias sputtering and characterization of LiCoO2 thin film cathodes for thin film microbattery. Mater. Chem. Phys. 2005, 93, 70–78. [Google Scholar] [CrossRef]
- Tintignac, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.-P.; Salot, R. High performance sputtered LiCoO2 thin films obtained at a moderate annealing treatment combined to a bias effect. Electrochim. Acta 2012, 60, 121–129. [Google Scholar] [CrossRef]
- Tintignac, S.; Baddour-Hadjean, R.; Pereira-Ramos, J.P.; Salot, R. Electrochemical properties of high rate bias sputtered LiCoO2 thin films in liquid electrolyte. J. Power Sources 2014, 245, 76–82. [Google Scholar] [CrossRef]
- Park, H.Y.; Nam, S.C.; Lim, Y.C.; Choi, K.G.; Lee, K.C.; Park, G.B.; Kim, J.B.; Kim, H.P.; Cho, S.B. LiCoO2 thin film cathode fabrication by rapid thermal annealing for micro power sources. Electrochim. Acta 2007, 52, 2062–2067. [Google Scholar] [CrossRef]
- Jeevan-Kumar, P.; Jayanth-Babu, K.; Hussain, O.M. Electrochemical performance of rf magnetron sputtered LiCoO2 thin film positive electrodes. AIP Conf. Proc. 2010, 1313, 224–226. [Google Scholar]
- Xie, J.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Cao, G.S.; Zhao, X.B. Amorphous LiCoO2 thin films on Li1+x+yAlxTi2−xSiyP3−yO12 prepared by radio frequency magnetron sputtering for all-solid-state Li-ion batteries. Electrochim. Acta 2010, 55, 5440–5445. [Google Scholar] [CrossRef]
- Kim, H.K.; Yoon, Y.S. Characteristics of rapid-thermal annealed LiCoO2 cathode film for an all-solid-state thin film microbattery. J. Vac. Sci. Technol. A 2004, 22, 1182–1187. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Vijh, A.; Zaghib, K. Lithium Batteries: Science and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Polo da Fonseca, C.N.; Davalos, J.; Kleinke, M.; Fantini, M.C.A.; Gorenstein, A. Studies of LiCoO2 thin film cathodes produced by r.f. sputtering. J. Power Sources 1999, 81–82, 575–580. [Google Scholar]
- Jang, Y.-I.; Dudney, N.J.; Blom, D.A.; Allard, L.F. Electrochemical and electron microscopic characterization on thin-film LiCoO2 cathodes under high-voltage cycling conditions. J. Power Sources 2003, 119–121, 295–299. [Google Scholar]
- Noh, J.P.; Jung, K.T.; Kwon, T.H.; Cho, G.B.; Huh, S.C.; Choi, B.K.; Kim, K.W.; Nam, T.H. Microstructure and electrochemical properties of magnetron-sputtered LiCoO2/LiNiO2 multi-layer thin film electrode. Mater. Res. Bull. 2013, 48, 4993–4996. [Google Scholar] [CrossRef]
- Bouwman, P.J.; Boukamp, B.A.; Bouwmeester, H.J.M.; Notten, P.H.L. Influence of diffusion plane orientation on electrochemical properties of thin film LiCoO2 electrodes. J. Electrochem. Soc. 2002, 140, A699–A709. [Google Scholar] [CrossRef]
- Jacke, S.; Song, J.; Cherkashinin, G.; Dimesso, L.; Jaegermann, W. Investigation of the solid-state electrolyte/cathode LiPON/LiCoO2 interface by photoelectron spectroscopy. Ionics 2010, 16, 769–775. [Google Scholar] [CrossRef]
- Matsushita, T.; Dokko, K.; Kanamura, K. Comparison of electrochemical behavior of LiCoO2 thin films prepared by sol-gel and sputtering processes. J. Electrochem. Soc. 2005, 152, A2229–A2237. [Google Scholar] [CrossRef]
- Matsushita, T.; Dokko, K.; Kanamura, K. In situ FT-IR measurement for electrochemical oxidation of electrolyte with ethylene carbonate and diethyl carbonate on cathode active material used in rechargeable lithium batteries. J. Power Sources 2005, 146, 360–364. [Google Scholar] [CrossRef]
- Matsui, M.; Dokko, K.; Kanamura, K. Dynamic behavior of surface film on LiCoO2 thin film electrode. J. Power Sources 2008, 177, 184–193. [Google Scholar] [CrossRef]
- Matsui, M.; Dokko, K.; Akita, Y.; Munakata, H.; Kanamura, K. Surface layer formation of LiCoO2 thin film electrodes in no-aqueous electrolyte containing lithium bis(oxalate)borate. J. Power Sources 2012, 210, 60–66. [Google Scholar] [CrossRef]
- Bai, L.; Xue, W.; Qin, H.; Li, Y.; Li, Y.; Sun, J. A novel dense LiCoO2 microcrystalline buffer layer on a cathode-electrolyte interface for all-solid-state lithium batteries prepared by the magnetron sputtering method. Electrochim. Acta 2019, 295, 677–683. [Google Scholar] [CrossRef]
- Madhavi, S.; Subba-Rao, G.V.; Chowdari, B.V.R.; Li, S.F.Y. Effect of Cr dopant on the cathodic behavior of LiCoO2. Electrochim. Acta 2002, 48, 219–226. [Google Scholar] [CrossRef]
- Zou, M.; Yoshio, M.; Gopukumar, S.; Yamaki, J. Performance of LiM0.05Co0.95O2 cathode materials in lithium rechargeable cells when cycled up to 4.5 V. Chem. Mater. 2005, 7, 1284–1286. [Google Scholar] [CrossRef]
- Sivajee-Ganesh, K.; Purusottam-Reddy, B.; Jeevan-Kumar, P.; Hussain, O.M. Influence of Zr dopant on microstructural and electrochemical properties of LiCoO2 thin film cathodes by RF sputtering. J. Electroanal. Chem. 2018, 828, 71–79. [Google Scholar] [CrossRef]
- Sivajee-Ganesh, K.; Purusottam-Reddy, B.; Hussain, O.M.; Mauger, A.; Julien, C.M. Influence of Ti and Zr dopants on the electrochemical performance of LiCoO2 film cathodes prepared by rf-magnetron sputtering. Mater. Sci. Eng. B 2016, 209, 30–36. [Google Scholar] [CrossRef]
- Needham, S.A.; Wang, G.X.; Liu, H.K.; Drozd, V.A.; Liu, R.S. Synthesis and electrochemical performance of doped LiCoO2 materials. J. Power Sources 2007, 174, 828–831. [Google Scholar] [CrossRef]
- Sivajee-Ganesh, K.; Purusottam-Reddy, B.; Jeevan-Kumar, P.; Jayanth-Babu, K.; Rosaiah, O.; Hussain, O.M. Microstructural and electrochemical properties of LiTiyCo1-yO2 film cathodes prepared by rf- sputtering. J. Solid State Electrochem. 2015, 19, 3621–3627. [Google Scholar] [CrossRef]
- Sivajee-Ganesh, K.; Purusottam-Reddy, B.; Jeevan-Kumar, P.; Jayanth-Babu, K.; Rosaiah, P.; Hussain, O.M. Structural and AC impedance analysis of rf sputtered Ti doped LiCoO2 thin films. Int. J. ChemTech Res. 2014, 6, 1974–1976. [Google Scholar]
- Moitzheim, S.; Put, B.; Vereecken, P.M. Advances in 3D thin-film Li-ion batteries. Adv. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Zadin, V.; Kasemägi, H.; Aabloo, A.; Brandell, D. Modelling electrode material utilization in the trench model 3D-microbattery by finite element analysis. J. Power Sources 2010, 195, 6218–6224. [Google Scholar] [CrossRef]
- Zadin, V.; Brandell, D.; Kasemägi, H.; Lellep, J.; Aabloo, A. Designing the 3D-microbattery grometry using the level-set method. J. Power Sources 2013, 244, 417–428. [Google Scholar] [CrossRef]
- Grazioli, D.; Verners, O.; Zadin, V.; Brandell, D.; Simone, A. Electrochemical-mechanical modeling of solid polymer electrolytes: Impact of mechanical stresses on Li-ion battery performance. Electrochim. Acta 2019, 296, 1122–1141. [Google Scholar] [CrossRef]
- Baggetto, L.; Niessen, R.A.H.; Roozeboom, F.; Notten, P.L.H. High energy density all-solid-state batteries: A challenging concept towards 3D integration. Adv. Func. Mater. 2008, 18, 1057–1066. [Google Scholar] [CrossRef]
- Ruzmetov, D.; Oleshko, V.P.; Haney, P.M.; Lezec, H.J.; Karki, K.; Baloch, K.H.; Agrawal, A.K.; Avydov, A.V.; Krylyuk, S.; Liu, Y.; et al. Electrolyte stability determines scaling limits for solid-state 3D Li ion batteries. Nano Lett. 2012, 12, 505–511. [Google Scholar] [CrossRef]














| Electrochemical Chain | Specific Capacity (µAh cm−2 µm−1) | Cyclability @ Current Density | Ref. |
|---|---|---|---|
| Li/LiPON/6.2 µm LCO/Pt/Ti/glass | 40 | 40@20 µA cm−2 | [22] |
| Li/1.5 µm LiPON/Pt/3.7 µm LCO | 67 | 50@200 µA cm−2 | [23] |
| Li/LiPON/0.5 µm LCO/Pt | 50 | 140@10 µA cm−2 | [24] |
| Li/1.4 µm LiPON/0.45 µm LCO/Au | 40 | [email protected] (10 µA cm−2) | [25] |
| Li/LiPON/NASICON/LCO/Pt | 15 | [email protected] | [26] |
| Li/Li2.64PO2.81N0.33/LCO/Pt/mica | 22 | 800@10C | [27] |
| Li/Li3.09BO2.53N0.52/LCO/Pt/mica | 44.3 | 1000@1C | [28] |
| Li/LiPON/LCO/Au/Ti/SiO2/Si | 58 | 30@4C | [29] |
| Li/LiPON/LCO/Pt/Ti/TiO2/Al2O3 | 60 | 500@5C | [30] |
| Composition | Target | N/P Ratio | Conductivity (S cm−1) | Ref. |
|---|---|---|---|---|
| Li4.2PO2.8N0.46 | Li3PO4 | 0.46 | 3.3 × 10−6 | [33] |
| Li4.2PO2.8N1.2 | Li3PO4 +Li3N | 1.2 | 4.1 × 10−7 | [34] |
| Li2.971PO1.875N1.25 | Li3PO4 | 1.2 | 1.67 × 10−6 | [35] |
| Li3.3PO2.1N1.4 | Li3PO4 | 1.4 | 1.6 × 10−6 | [36] |
| Li2.9PO2.9N0.5 | Li3PO4 | 0.5 | 1.4 × 10−6 | [37] |
| Li4.0PO3.9N0.4 | Li3PO4 | 0.4 | 1.75 × 10−6 | [38] |
| Li3.2PO3.0N1.0 | Li3PO4 | 1.0 | 3.0 × 10−6 | [38] |
| Atmosphere a) | Power (W) | Deposition Rate (nm s−1) | Substrate | Substrate Temperature (°C) | Structural/Electrochemical Properties c) | Ref. |
|---|---|---|---|---|---|---|
| 3:1/55/1.0 | 100 | 3.2 | Si (100) wafer | 25 | Influence of the target history and deposition geometry | [51] |
| 9:3/12/5 | 100 | Si/SiO2/Ti/Pt | 250 | Ta = 700 °C, Qd = 61 µAh cm−2 µm−1; Rc = 74% after 50 cycles | [63] | |
| 3:1/40/0.5 | 80 | 1.6 | Si/Ti/MgO/Pt | 10 | Ta = 800 °C, Qd = 70 µAh cm−2 µm−1 @ 5 µA cm−2; Rc = 30% over 40 cycles | [64] |
| 96:4/50/0.5 | 2.75 b) | ~0.3 | Al2O3/Ti/Au | ~110 | Ta = 800 °C, Qd = 60 µAh cm−2 µm−1 @ C/10; Rc = 95% over 100 cycles | [47] |
| 9:1/-/0.5 | 4.4 b) | Si/Pt and Cu foil | 200 | Ta = 700 °C, Qd = 52 µAh cm−2 µm−1 @ 50 µA | [61] | |
| 5:1/150/0.2 | 130 | 0.03 | Al foil | 65 | c, Qd = 46 µAh cm−2 µm−1 @ 5 µA cm−2; Rc = 78% over 100 cycles | [65] |
| 9:1/-/0.5 | 150 | 0.1 | Si/Al/Li2O | 25 | (101)-oriented; Qd = 40 µAh cm−2 µm−1@20 µA cm−2; Rc = 78%@640 µA cm−2 | [48] |
| 4:1/150/0.27 | 130 | 0.05 | Stainless steel | 25 | Qd = 44 µAh cm−2 µm−1@10 µA cm−2; Rc = 66% after 30 cycles | [66] |
| 3:1/53/2.2 | 500 | Al foil | 25 | Ta = 500 °C, Qd = 50 µAh cm−2 µm−1@10 µA cm−2; Rc = 80% after 800 cycles | [25] | |
| 1:0/-/2.0 | 100 | 8.3 | Au | 25 | Kinetics of (104)-plane. DLi ≈ 10−10–10−12 cm2 s−1 | [67] |
| 2:1/-/0.5 | 200 | Pt wafer | 55 | Power of 200 W, Qd = 61 µAh cm−2 µm−1@20 µA cm−2 | [68] | |
| 40:1/20/0.14 | 500 | 1 | Quartz/Pt | 300 | Thickness dependence; Qd = 72 µAh cm−2 µm−1@0.1 mA cm−2 | [23] |
| 3:1/12/2 | 100 | Si/Pt | 25–600 | Ts = 250 °C, Ta = 600 °C, Qd = 50 µAh cm−2 µm−1@10 µA cm−2 | [70] | |
| 9:1/-/0.5 | 50 | 0.8 | Sapphire/SiO2/Al | 25 | Ta = 500 °C, thermal conductivity 3.7 W m−1 K−1 for Li0.6CoO2 | [71] |
| 9:3/12/0.5 | 50 | 0.02 | Si/SiO2/Pt | 25 | Ta = 800 °C, Qd = 27 µAh cm−2 µm−1@50 µA cm−2 after 150 cycles | [72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Julien, C.M.; Mauger, A.; Hussain, O.M. Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries. Materials 2019, 12, 2687. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12172687
Julien CM, Mauger A, Hussain OM. Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries. Materials. 2019; 12(17):2687. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12172687
Chicago/Turabian StyleJulien, Christian M., Alain Mauger, and Obili M. Hussain. 2019. "Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries" Materials 12, no. 17: 2687. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12172687