3.1. Effect of Temperature and CO2 on the Corrosion Rate
To have a general overview on the effect of solution solely (without gases) and with different gases on the corrosion behaviors of carbon steel, exposure tests were performed for seven days in three conditions: deaerated with Ar, aerated, and CO
2-containing solutions at 25, 70 and 150 °C.
Figure 1 shows the corrosion rate of carbon steel as a function of temperature after seven-day exposure tests. For industrial application, the corrosion rate threshold is usually set to 0.3 mm/year to ensure the safety of material constructions, albeit with the requirement that there is no localized or pitting corrosion observed [
7]. As shown in the results of deaerated tests, although the solution contains 1500 mg/L chloride, Sibayak solution alone did not lead to a corrosion rate of more than 0.1 mm/year, regardless the testing temperatures. When dissolved oxygen is present, as simulated by the aerated solution, the corrosion rate significantly increases with temperature, suggesting that dissolved oxygen contributes to accelerating corrosion reaction. This result is in agreement with previous studies and confirms the presence of oxygen to be the main cause of corrosion rate increase [
9,
31,
32]. In the geothermal water medium, 30 ppb of oxygen causes up to four times increase of corrosion rate on carbon steel, while the presence of oxygen with concentrations higher than 50 ppb causes serious pitting corrosion [
32,
33]. Although dissolved oxygen decreases with the increase of temperature, there is a possibility that oxygen traces intrude to a certain depth of a geothermal well and cause corrosion [
3,
34,
35].
Beside oxygen that may exist with a low concentration, CO
2 presents in Sibayak geothermal system as dissolved gas with a stream of 80–90 mol.% [
13]. Basing on the results of weight loss determination, under the presence of CO
2 at 70 °C and 150 °C, corrosion rates were 0.16 and 0.24 mm/year, respectively. Similar to the corrosion rates of carbon steel in the presence of oxygen, corrosion rates of CO
2 exposed specimens increase with temperature, but somewhat with a lower degree than in an oxygen-containing environment. The macro-photos and SEM images of tested specimens in the CO
2 environment (
Figure 1) show a very homogeneous and dense layer of corrosion products indicating uniform corrosion. For long-term exposure, the nature of corrosion products governs the continuity of corrosion processes. A resistive and dense layer of highly crystalline corrosion products will act as a protective barrier, while a porous layer might allow selective ions to penetrate through the metal surface, causing further corrosion processes. Therefore, it is necessary to investigate to what extent CO
2 does influence the corrosion of carbon steel and the nature of its corrosion products.
3.2. Effect of Temperature on Corrosion Product Formation
To identify the morphology and characteristics of the main corrosion products formed on the carbon steel surface, SEM images of carbon steel surface were taken.
Figure 2 shows a higher magnification of SEM images after the carbon steel was exposed to 70 °C and 150 °C in CO
2 and Ar condition for seven days. In conjunction with the surface morphology images, additional information is provided, such as the cross-section, elemental composition, and phases, as shown in
Figure 2.
In the CO
2-saturated condition at 70 °C (
Figure 2a), there are two dominant crystal structures, plate-like and prismatic-shaped crystals. X-ray diffraction data (
Figure 2b) showed that the corrosion products were predominantly Fe
2(OH)
2CO
3 chukanovite (COD 9010838) and FeCO
3 siderite (COD 9000098). The plate-like crystal structure may be then assigned to chukanovite, whereas the prismatic cubic shape crystal is a characteristic of siderite crystal structure, as also reported in other studies [
36,
37,
38]. Chukanovite exists as a metastable phase, commonly found in a laboratory scale experiment instead of in the realistic field condition. This corrosion product does not adhere to the carbon steel surface, which leads to poor protectiveness. In CO
2-saturated solution at 70 °C, siderite has a thickness of 10–22 µm, and chukanovite has a thickness of 5–18 µm. Underneath the corrosion product layer, localized corrosion was found with the depth of 6–15 µm (
Figure 2c). As can be seen in
Figure 2a, chukanovite was mixed with siderite and did not completely cover the carbon steel surface. Consequently, the resulting space between those two types of crystal structure opened pathways for the electrolyte to further react with the carbon steel surface (
Figure 2c). Therefore, carbon steel in this condition is susceptible to a pit initiation. Without CO
2, as represented by experiments under Ar, the thickness of the corrosion product layer is significantly reduced, and the products did not cover the entire surface (
Figure 2d). The brighter area in the SEM image shows the iron surface that is not fully covered by the corrosion product. This result shows that at 70 °C more accumulation of corrosion product was observed in the CO
2-containing solution (
Figure 2a) than in the deaerated solution (
Figure 2d), which confirms the corrosive effect of CO
2 toward carbon steel in geothermal solution (
Figure 1).
At 150 °C, the prismatic structure was observed as the dominant corrosion product (
Figure 2e) identified as FeCO
3. The appearance of a very homogeneous structure, hence the absence of the plate-like crystal structure, which was previously identified as chukanovite, showed that siderite formation is more favorable at 150 °C. Similar observations were also confirmed by other studies [
22,
39]. Saturation degree (S) is a key parameter in the formation and precipitation of corrosion products, depending on the ionic concentration (c) and solubility limit (K
sp). For example, for FeCO
3:
When the concentration of Fe2+ and CO32− increases, more FeCO3 precipitates and crystalizes on the surface. At 70 °C, FeCO3 was formed; however, this temperature is not high enough to form the dense and protective FeCO3. Thus, the experimental results evidently showed that the precipitation and formation of FeCO3 are highly favored at a higher temperature.
To confirm the FeCO
3 formation on the carbon steel surface at 150 °C, EDX was used to identify the constituent elements of the corrosion product. Several points were selected and analysis by EDX (
Figure 2f) revealed not only Fe, C, and O, but also a small amount of Ca. The detected Ca element can be associated with the incorporation of Ca
2+ ion into the FeCO
3 structure, due to the isostructural behavior of CaCO
3 and FeCO
3 [
40]. EDX results showed the atomic ratio of Ca and Fe, leading to x = 0.98 (±0.01) and y = 0.02 (±0.01) for a mixed carbonate compound Fe
xCa
yCO
3. In an extended exposure test, the EDX spectra showed that the atomic ratio between Ca and Fe changed, resulting in x = 0.96 and y = 0.04. This change indicated that Ca
2+ can further diffuse to the FeCO
3 structure when the specimen is exposed with a longer time.
The incorporation of Ca
2+ in this experiment (at 150 °C, with 200 ppm Ca
2+) is considerably low compared to another study where 100 ppm Ca
2+ was used in stagnant condition at 80 °C, resulting in a y value of 0.22 [
41]. It is also noteworthy to mention that different brine chemistry affects the incorporation of Ca
2+ to the FeCO
3 structure, as the experiment was not performed in a pure CaCl
2 solution, but rather in an artificial geothermal brine having various ionic species. Additionally, this study uses higher temperature and stagnant solution at 150 °C. As concluded by Mansoori et al. [
40], it is still debatable whether Ca
2+ has a positive or negative influence on the corrosion behavior of different brine chemistry and experimental conditions.
At 150 °C, a thickness of the corrosion product layer was measured between 9–27 µm (
Figure 2g). There was also a take-up of carbon steel of 5–8 µm, which may be associated with the iron dissolution in the initial stage of exposure, followed by FeCO
3 formation. Without CO
2 gas, corrosion products have very small crystal size around 1 µm (
Figure 2h), which is 60 times smaller than that observed in the CO
2 environment (
Figure 2e), which indicate that the type of dissolved gas influences the corrosion processes.
As suggested by Tanupabrungsun et al. [
27], there is a possibility that at 150 °C FeCO
3 and magnetite (Fe
3O
4) are formed simultaneously. This phenomenon was simulated by a Pourbaix diagram of a Fe-CO
2-H
2O system at 150 °C with 10 ppm Fe
2+ and 10 ppm Fe
3+, which was also proven by an experimental work. Given this possibility, EBSD analysis was performed to ensure the composition of the corrosion products more precisely.
Figure 3 shows the EBSD mapping of the carbon steel cross section, in which carbon steel was exposed to the CO
2-containing geothermal water at 150 °C for seven days.
Based on the EBSD mapping, the phase composition of the cross section consists of siderite on the top layer and iron on the bottom layer as the base material (
Figure 3b). This result confirmed that in this experimental condition, FeCO
3 was formed at 150 °C without the concurrence of Fe
3O
4 formation. In addition, the crystal orientation map shows that the grain size of siderite varies from about 10–60 µm (
Figure 3c–e), which is about similar to the crystal size of FeCO
3, as observed from the top view of the specimen using SEM (
Figure 2e).
Longer exposure tests were performed for 28 days to investigate the growth and stability of the corrosion product. After 28 days of exposure, surface inhomogeneity was observed by macro-photography (inserted in
Figure 4a). The brighter area had a different morphology than that of the seven-day exposed specimen, whereas the darker area consisted of FeCO
3 with an identical structure as
Figure 2e. Within the brighter area, the corrosion products had smoother crystal, and there are some smaller prismatic structures (
Figure 4a). Although the corrosion product was protective, the cross-section revealed localized pits of up to 72 µm (
Figure 4b) with a reduction of the FeCO
3 thickness to the range of 6–13 µm, compared to that of the seven-day exposed specimens. EDX line scanning of the specimen cross-section revealed the distribution of elements within the layer and the pit presented by line scan 1 and line scan 2, respectively (
Figure 4c). Here, carbon composition is not shown due to the sample preparation using carbon sputtering. Ca was detected in the entire layer thickness (line scan 1) for about 12 µm, whereas within the pit, Ca was diminished after about 40 µm depth (line scan 2). Thus, it can be concluded that the diffusion of Ca is limited to a certain depth.
Figure 4d shows that in deaerated solution, carbon steel was entirely covered by a homogeneous and dense layer of corrosion products consisting of two distinct morphologies, i.e., prismatic and needle-like structure. When compared to the carbon steel surface after seven days of exposure (
Figure 2h), the crystal structure has a more dense and bigger structure, indicating a continuous growth along exposure time. Although having a denser crystal structure, the corrosion product layer was not protective, where several pits were observed with pit depth of 3 µm after 28 days of exposure (
Figure 4e). Pitting corrosion was not only observed in the presence of CO
2 but also in the absence of CO
2; however, to a less degree than when CO
2 is present in the system.
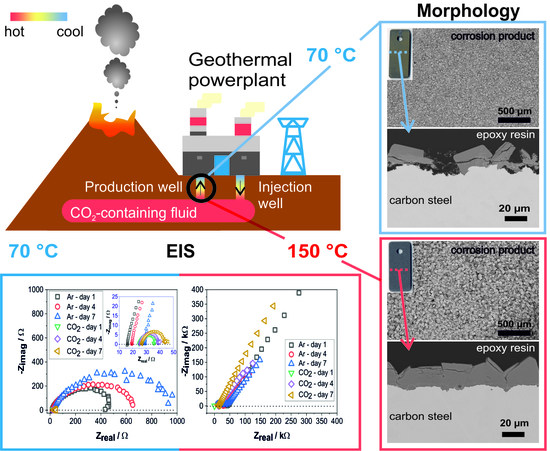
3.3. Effect of CO2 on the Corrosion Behavior
To study the effect of CO2 on the corrosion behavior of carbon steel in geothermal brine, electrochemical measurements, including open circuit potential and impedance spectroscopy, were performed both in a deaerated solution and a CO2-containing solution.
Open circuit potential was recorded during exposure time to trace the corrosion reactions. The results were then selected at 24 h interval and plotted as E
corr versus time, as shown in
Figure 5. At 70 °C, E
corr was between −693 to −701 mV for the specimen exposed to the deaerated solution (
Figure 5a). E
corr was stable during the entire exposure, giving a maximum deviation of 8 mV, indicating a constant interaction of carbon steel surface with the brine without a significant change of the electrochemical reaction. As shown in
Figure 2d, only a small amount of corrosion product is accumulated on the carbon steel, which then allows the brine to react directly with the metal surface, therefore it remains actively corroded. Similarly, E
corr of the specimens exposed to CO
2-containing solution exhibited a small increase from −669 mV to −648 mV along the seven days of exposure. The most significant increase in E
corr was observed within 24 h, where the potential increased by 11 mV, suggesting a fast-initial dissolution of Fe
2+ from the metal surface, followed by almost unchanged formation kinetics of the corrosion products.
Electrochemical tests were then performed at 150 °C to reveal the effect of temperature on the corrosion process. Without CO
2, there was a significant increase of E
corr after 24 h of exposure, from -457 mV to 39 mV, which then gradually decreased, reaching −206 mV after seven days. This result showed that in the first 24 h, a more cathodic reaction took place between Fe
2+ and H
2O, influenced by high applied temperature [
42]. The gradual decrease of E
corr indicates a continuous active corrosion process after 24 h, albeit with a less degree compared to that at 70 °C.
In the CO
2-containing solution, E
corr increased significantly compared to the E
corr observed at 70 °C with CO
2 gas, although not as significantly as at 150 °C in the deaerated solution in the first 24 h. The E
corr increase can be divided into two steps. The first increase was within the first 24 h, where the E
corr increased from −662 mV to −510 mV, giving an increase of 152 mV. The second increase of potential was with a slower rate, reaching saturation of E
corr on the fifth day of exposure (−335 mV), which corresponds to the growth of FeCO
3 layer. As shown in
Figure 4b, there was no significant difference in the potential between the fifth and seventh days of experiments. at 150 °C; unlike the OCP observed in the deaerated solution, E
corr increases gradually under the CO
2 condition, indicating the formation of a passive layer and contributing to a more cathodic potential.
Further investigation was performed by EIS to characterize the surface-interface interaction of carbon steel, electrolyte, and the corrosion products. Impedance data are presented in different formats to distinguish different specific behaviors. Complex-impedance-plane representation, usually also known by Nyquist plot, indicate the possible mechanism by the shape of the position of the points at each frequency. However, it does not explicitly show information about frequency; hence, the Bode Plot and Phase Angle plot provides more detailed information. Bode representation depicts the absolute impedance value with respect to frequency, whereas the Phase Angle plot shows the phase angle with respect to frequency.
Figure 6 shows a comparison of the Nyquist plots from the exposed specimen between the deaerated and CO
2-containing solutions. For both conditions, the Nyquist plot showed results on the negative value of Z
imag (y-axis), indicating a contribution of a capacitive reactance to the impedance value.
At 70 °C, the impedance of specimen exposed to the deaerated solution exhibits a higher corrosion resistance than in the CO
2-containing solution, indicated by a bigger diameter of the capacitive semicircles of more than 20 times (
Figure 6a). Overall, the diameter of capacitive semicircle observed in both the deaerated and CO
2-containing solution significantly increased over time, indicating a higher charge transfer resistance (R
ct) over time, and therefore slower reaction kinetics on the surface. After seven days, the diameter of a capacitive semicircle observed in the deaerated solution was almost 50 times higher than in CO
2-containing solution. By evaluating the change of Nyquist plot (
Figure 6a) with an interval of three days, a significant increase was observed of almost 200 Ω in the deaerated solution and around 5 Ω in the CO
2-containing solution. Although E
corr at 70 °C did not directly show the influence of CO
2 (
Figure 5), the EIS data evidently showed that carbon steel is more prone to corrosion in the CO
2-containing solution than in the deaerated solution.
The Nyquist plot showed an exponentially increased impedance of specimens at 150 °C (
Figure 6b), compared to that at 70 °C (
Figure 6a). Despite the higher corrosion rate of specimen exposed to the CO
2-containing solution compared to that exposed to the deaerated solution at 150 °C (
Figure 1), the Nyquist plot showed an indistinct high corrosion resistance of carbon steel in both solutions (
Figure 6b). Instead of a capacitive semicircle, the Nyquist plot showed a diagonal line with a positive slope. Due to the limited frequency range, it is difficult to verify whether this pattern belongs to a much bigger capacitive semicircle, or is solely based on diffusion and mixed kinetic processes. To further interpret the data, additional information was analyzed based on the Bode plot, which has an advantage of presenting the total magnitude of impedance value and the phase angle as a function of frequency (
Figure 7 and
Figure 8).
3.4. Scaling Formation and Growth at Different Temperatures in a CO2 Environment
As revealed by OCP (
Figure 5) and EIS data (
Figure 6) in the previous section, the interdependency of free corrosion potential (OCP) and physical-electrical behavior (EIS) is not always straightforward to be interpreted. For example,
Figure 5 showed an almost unchanging OCP of carbon steel at 70 °C, whereas
Figure 6a showed a significant increase in the impedance of the corresponding specimen. As also discussed in the corrosion potential monitoring, the most important spontaneous reaction at 150 °C occurred within the first day (
Figure 5), which necessitates a more precise EIS analysis within this initial stage to further analyze the kinetics of iron dissolution and the corrosion product formation. Thus, EIS data was recorded every 2 h in the first 12 h of exposure in addition to the day-to-day measurement.
Figure 7 presents the evolution of impedance spectra in all three types of plots—Nyquist, Bode and phase angle plots—for each dataset, where carbon steel is exposed to the CO
2 containing solution at 70 °C for seven days.
Although there was only a very small change in free corrosion potential of carbon steel at 70 °C (
Figure 5b), impedance spectra did show changes with respect to time. Within seven days of exposure, impedance spectra showed that the capacitive semicircles had diameter of less than 20 Ω, indicating a fast charge-transfer process on the surface of the tested specimen at 70 °C due to reactions with the CO
2-containing artificial brine solution. Regardless of the stable impedance value over seven days, the impedance spectra did show two main stages of change: a decrease of impedance in the first day (
Figure 7a–c); and a continuous, but slow increase of impedance from the first to seventh day (
Figure 7d–f).
During the first 24 h, impedance decreases, as shown in
Figure 7a,b. Phase angle remained at around −9° at a high-to-medium frequency range, indicating that the characteristics of the charge transfer process are not capacitive, mainly because of metal dissolution. It is suggested that, on the first day, the fresh ground metal surface reacted with the solution, where selective dissolution of ferrite took place, and Fe was dissolved along with hydrogen evolution [
21]. Low carbon steel is dominated by ferrite microstructure (α-Fe) with a secondary microstructure of cementite (Fe
3C). As the ferrite dissolved, cementite remained on the surface, providing preferential cathodic sites with lower overpotential favoring hydrogen evolution [
43]. This process resulted in the increment of charge-transfer processes and therefore the decrease of the characteristic semicircle diameter. In a similar test using carbon steel C1018 and deaerated 3% NaCl with pH 6 at 80 °C, Farelas et al. found a decrease of capacitive semicircle diameter until 15 h of exposure [
21], which is comparable to this work, where a decrease of semicircle was also observed until 12 h of exposure.
From the second day onwards (
Figure 7d), the diameter of capacitive semicircles increased with respect to time. The dissolved Fe
2+ formed corrosion products on the surface, as revealed by SEM image after seven days of exposure (
Figure 2a). Although it covered the surface, the corrosion product was not strongly attached, allowing the metal to actively corrode, and therefore resulting in only a small increase of impedance. At frequency 10
−1 to 10
−2 Hz,
Figure 7e shows that the magnitude of impedance slightly increased. The peak of the phase angle changed to a more negative value, indicating an increase of capacitance, which might be associated with the increase of surface coverage by the corrosion product. Consequently, the peak of phase angle shifted to a lower frequency, indicating slower kinetics of metal dissolution with respect to time. The increase of surface coverage by the corrosion product might hinder the mass transport towards the surface. Thus, the overall impedance was the highest by the end of the seventh day, indicating a higher corrosion resistance, although not to a significant extent.
At the lower frequency range, the Nyquist plot showed a small loop that took place both in the early stage of the first day (
Figure 7a) and the later stage (
Figure 7d). An inductive loop at low frequency is often observed, where CO
2 was used in 3 wt.% NaCl solution [
21], associated with reactive ionic species or adsorption of intermediate products. However, in this case, the loop was neither a distinct second semicircle (capacitive loop) nor an inductive loop at y-axis below 0 Ω (dotted line), which rather suggested an additional capacitive effect, instead of a simple inductive loop interpretation.
A capacitive and/or inductive loop may deviate when an intermediate reaction occurs, e.g., dielectric relaxation, thickness modulation in the porous layer, and frequency-dependent impedance [
44,
45]. Frequency-dependent impedance is related to capacitive behavior, since a capacitor can charge and discharge at different frequency. One possible reason of capacitive and inductive loop deviation is the reaction between metal surface and the complex tested solution, i.e., 1500 mg/L of Cl
−, 20 mg/L of SO
42−, and 15 mg/L of HCO
3−. The role of Cl
− and SO
42− on the iron dissolution mechanism has been studied by Barcia et al. [
46], and it was shown that at pH 3 and 4 with an anodic polarization of 0.05 A/cm
2, inductive loop in the lower frequency changes to capacitive loop. The same behavior was also observed by Bechet et al. [
47] in sulfuric acid medium with pH 2.6 and anodic polarization of 100 mA/cm
2. As HCO
3− is also present due to the deprotonation of hydrated CO
2, having HCO
3− in the solution does not seem to have a significant influence on the reaction.
As shown in
Figure 5 and
Figure 6b, there were significant changes not only in the E
corr but also in the impedance of specimens exposed at 150 °C, as compared to that at 70 °C, indicating a strong influence of temperature on the corrosion behavior. Similar measurement interval as that at 70 °C was applied at 150 °C to observe the early stage reaction within 24 h. The Nyquist plot of carbon steel exposed to 150 °C during the first few hours (2–24 h) revealed that electrolyte resistance was around 3.5 times lower than at 70 °C, i.e., 7 Ω, due to the increased temperature, resulting in a higher ionic mobility (
Figure 8a).
Between 2–12 h, the Nyquist plot does not present a single semicircle as that observed at 70 °C, but it does not distinctively show the second semicircle, resulting in the complexity in interpreting the exact underlying mechanism of corrosion in the first few hours. The Bode magnitude plot shows that there was an increase in absolute impedance value between 2–4 h, which then slightly decreased until 12 h, and then again increased at 24 h. Similar to that at 70 °C, there are an indication of two time-constants between 0–12 h (
Figure 8c), albeit with a more complex phase angle graph with a second time constant (5 Hz–0.1 Hz) that cannot be interpreted assuredly. Compared to 70 °C, EIS data (
Figure 8a–c) showed more varying patterns within 24 h, indicating more complex, unstable, and rapidly evolved processes.
Figure 8d shows a Nyquist plot between 1–7 days, where a significant increase of impedance was observed in comparison with the first 24 h (
Figure 8a). The total impedance increase was around three orders of magnitude, as also shown in the Bode magnitude plot (
Figure 8e). The high impedance value is usually associated with the passive behavior of the corrosion product layer. After the second day, the phase angle shifted to a much lower frequency, suggesting slower kinetics of interfacial reactions. The change of phase angle to a more negative value may also be associated with an increase of corrosion product coverage on the surface. All the data representation showed a stable pattern at a very high resistance, indicating that the FeCO
3 is protective between the second and seventh day.
Considering all the corrosion processes indicated by the OCP, EIS, and surface analyses data, equivalent electrical circuits are suggested for different temperatures and corrosion stages as inserted in
Figure 7a,d and
Figure 8a,d. R
s is solution resistance, Q
dl is a constant phase element representing double layer capacitance, and R
ct is charge transfer resistance. At 150 °C, additional components were added to the equivalent electrical circuit (
Figure 8d). Q
c is a constant phase element representing the corrosion product layer capacitance and R
po is the corrosion product layer resistance.
Here, Q represents constant phase element (CPE), which is assumed to have originated from the local surface distribution of reactivity. When the electrochemical reaction is coupled by an adsorbed intermediate, the non-uniform current and potential distribution may result in an increase of a low-frequency dispersion [
48]. CPE parameters consist of Q and α, which are representative of a physical system. Here, capacitance is not calculated from Q and α because an interpretation of CPE impedance response to estimate either dielectric constant or film thickness in high precision requires a value for resistivity at the film-electrolyte interface, ρ
δ [
49]. However, R
ct, also often interpreted as corrosion resistance or polarization resistance (R
p), was extracted to show the approximation of corrosion resistance as a function of time. Fitting result has the value of α between 0.5 and 1 with the goodness of fit (GoF) below 7.10
−4 representing the maximum error in data fitting between 1–10%.
3.5. Corrosion Mechanism
The corrosion rate of carbon steel in geothermal water is influenced by the gas content, with the order of corrosivity level: O2 presence > CO2 presence > deaerated/Ar, as confirmed by the above-presented results. By comparing the specimen exposed to CO2 and Ar-containing solution via exposure and electrochemical tests, the corrosive effect of CO2 was proven to be more significant. SEM-EDX was further used to confirm the corrosion behavior of carbon steel after the test was carried out.
At 70 °C, corrosion products were dominated by Fe
2(OH)
2CO
3 and FeCO
3, whereas at 150 °C, it was dominated by only FeCO
3 (
Figure 2), which shows that FeCO
3 formation is more favorable at 150 °C. The different morphology of corrosion products can also affect the physical and electrochemical behavior of the tested specimens, and therefore the corrosion mechanism at different temperatures. At 70 °C, although there was no significant change in E
corr, impedance spectra of carbon steel exposed for seven days to the deaerated solution is almost 50 times higher than that of the one exposed to the CO
2-containing solution, which evidently confirmed the corrosive effect of CO
2.
At 150 °C, the impedance spectra of carbon steel exposed for seven days was not significantly different with or without presence of CO2, due to the faster formation of corrosion products in the first seven days in both conditions. However, Ecorr showed a higher increase of carbon steel free corrosion potential when exposed to the Ar-containing solution, compared to that exposed to the CO2-containing solution, indicating that carbon steel is more susceptible to corrosion under CO2 environment. Since the seven-day exposure test was not sufficient to distinguish the corrosive effect of the CO2-containing solution (as shown by Ecorr) from the protective effect of FeCO3 layer (as shown by EIS and SEM), a longer exposure test was performed to observe the corrosion behavior and the stability of FeCO3 at 150 °C. Morphological observation using SEM and EDX showed that after 28 days, the FeCO3 structure evolved and transformed. Pitting corrosion was observed with a depth up to 72 µm in the CO2 presence, and 3 µm without the CO2 presence, which further elucidate the corrosive effect of CO2 at 150 °C. Additionally, there was more Ca precipitation observed after 28 days, which might also affect the stability and corrosion protection of FeCO3.
It is important to note that the underlying corrosion mechanism for 70 °C and 150 °C is different, given that carbon steel remained actively corrode at 70 °C, whereas at 150 °C, there was a protective effect of FeCO
3 seen by EIS in seven days. Therefore, the possible corrosion mechanism is proposed separately by plotting polarization resistance (R
p) against time for results acquired at 70 °C (
Figure 9) and at 150 °C (
Figure 10).
At 70 °C, R
p decreases within the first 12 h, associated with the iron dissolution and hydrogen reduction reaction. After 12 h, R
p increases over time, indicating a formation of the corrosion products, i.e., Fe
2(OH)
2CO
3 and FeCO
3. All R
p observed until the seventh day was between 10–20 Ω and remained in the active region, suggesting a non-protective behavior of corrosion products. Although the formation and growth of FeCO
3 and Fe
2(OH)
2CO
3 was evidently shown by SEM and XRD (
Figure 2a,b), pitting corrosion was also observed in the cross section (
Figure 2c) with an initial pitting propagation in the area where the corrosion products are not adherent. Although there was small change in impedance spectra, it was not significant for both OCP and EIS, indicating that the main electrochemical reaction corresponds to the continuous reacting metal surface and the brine, regardless of corrosion product formation and growth.
At 150 °C, R
p remains below 100 Ω, which is considered stable in the active region until 24 h. There was a steep increase of R
p between 24 and 48 h, where polarization resistance increased exponentially at about five orders of magnitude, which showed a passive behavior, possibly due to the formation of a dense FeCO
3 layer. This result indicated a faster crystallization of FeCO
3 at 150 °C in only two days of exposure. After seven days, the corrosion product layer yielded a dense, compact, and adherent layer of FeCO
3 with a thickness between 9–27 µm, as proven by the SEM image of the cross section (
Figure 2f). The high R
p remained at the same range until seven days of exposure, which suggests that the FeCO
3 formed at 150 °C in the presence of CO
2 can act as a physical non-conductive barrier layer. Thus, it is clear that within two days of exposure, rapid corrosion processes take place at 150 °C, but require a longer time to validate whether FeCO
3 is protective or not. However, at 150 °C, all experiments were carried out in the autoclaves with a limitation that under such a temperature and pressure, CO
2 gas cannot be circulated continuously. Consequently, the corrosion mechanism at 70 °C cannot be compared directly to that at 150 °C, although the corrosive effect of CO
2 was evidently observed at both temperatures.
Given the above-discussed results, there is room for improvement, in terms of investigating other effects regarding the corrosion behavior of carbon steel in the CO2-containing geothermal solution, in particular, the effect of Ca2+ content in the solution, and the continuous supply of CO2 gas at high temperatures.