Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Formation of Photoelectrochemically Active Tungsten Oxide Layers on the FTO and Glass Substrate
2.3. Characterization of Oxide Layers
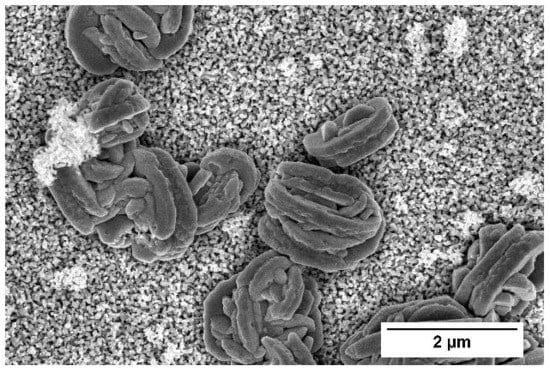
2.3.1. X-ray Diffraction, Scanning Electron Microscopy
2.3.2. Electrochemical Measurements
2.3.3. Time-Resolved Photoluminescence Decay Based Evaluation of WO3-Based Coatings
3. Results and Discussion
3.1. XRD, SEM and FTIR Analysis of WO3 Coatings
3.2. Time-Resolved Photoluminescence Decay Based Evaluation of WO3 Coatings
3.3. Photoelectrochemical Evaluation of WO3 Coatings Deposited on FTO-Glass Electrode
4. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, F.; Ho, H.-P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tereshchenko, A.; Bechelany, M.; Viter, R.; Khranovskyy, V.; Smyntyna, V.; Starodub, N.; Yakimova, R. Optical biosensors based on ZnO nanostructures: Advantages and perspectives. A review. Sens. Actuators B Chem. 2016, 229, 664–677. [Google Scholar] [CrossRef]
- Tereshchenko, A.; Smyntyna, V.; Ramanavicius, A. Interaction mechanism between TiO2 nanostructures and bovine leukemia virus proteins in photoluminescence-based immunosensors. RSC Adv. 2018, 8, 37740–37748. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Tang, H.; Hu, Q.; Zheng, S.; Li, L.; Xu, J.; Pang, H. Tungsten-Based Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1707500. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Berggren, L.; Larsson, A.-L. Electrochromic tungsten oxide: The role of defects. Sol. Energy Mater. Sol. Cells 2004, 84, 315–328. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J. Tungsten Oxides for Photocatalysis, Electrochemistry, and Phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef]
- Nath, N.C.D.; Choi, S.Y.; Jeong, H.W.; Lee, J.-J.; Park, H. Stand-alone photoconversion of carbon dioxide on copper oxide wire arrays powered by tungsten trioxide/dye-sensitized solar cell dual absorbers. Nano Energy 2016, 25, 51–59. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, J.; Liu, H.; Murugadoss, V.; Zu, G.; Che, H.; Lai, C.; Li, H.; Ding, T.; Gao, Q.; et al. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. Nanoscale 2019, 11, 18968–18994. [Google Scholar] [CrossRef]
- Sivakarthik, P.; Thangaraj, V.; Parthibavarman, M. A facile and one-pot synthesis of pure and transition metals (M = Co & Ni) doped WO3 nanoparticles for enhanced photocatalytic performance. J. Mater. Sci. Mater. Electron. 2017, 28, 5990–5996. [Google Scholar] [CrossRef]
- Gao, W.-B.; Ling, Y.; Liu, X.; Sun, J.-L. Simple point contact WO3 sensor for NO2 sensing and relevant impedance analysis. Int. J. Miner. Met. Mater. 2012, 19, 1142–1148. [Google Scholar] [CrossRef]
- Juodkazytė, J.; Šebeka, B.; Savickaja, I.; Petrulevičienė, M.; Butkutė, S.; Jasulaitienė, V.; Selskis, A.; Ramanauskas, R. Electrolytic splitting of saline water: Durable nickel oxide anode for selective oxygen evolution. Int. J. Hydrogen Energy 2019, 44, 5929–5939. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, C.; Zheng, L.; Zheng, W.; Liu, X.; Kumar, M.; Zhang, J. Highly sensitive and selective detection of acetone based on platinum sensitized porous tungsten oxide nanospheres. Sens. Actuators B Chem. 2020, 307, 127616. [Google Scholar] [CrossRef]
- Gao, P.; Ji, H.; Zhou, Y.; Li, X. Selective acetone gas sensors using porous WO3–Cr2O3 thin films prepared by sol–gel method. Thin Solid Films 2012, 520, 3100–3106. [Google Scholar] [CrossRef]
- Navarrete, E.; Bittencourt, C.; Umek, P.; Llobet, E. AACVD and gas sensing properties of nickel oxide nanoparticle decorated tungsten oxide nanowires. J. Mater. Chem. C 2018, 6, 5181–5192. [Google Scholar] [CrossRef]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.F.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Zhang, C.; Boudiba, A.; De Marco, P.; Snyders, R.; Olivier, M.-G.; Debliquy, M. Room temperature responses of visible-light illuminated WO3 sensors to NO2 in sub-ppm range. Sens. Actuators B Chem. 2013, 181, 395–401. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2-x/TiO2-Structure Based ’Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2019, 20, 74. [Google Scholar] [CrossRef] [Green Version]
- Dilova, T.; Atanasova, G.; Dikovska, A.O.; Nedyalkov, N. The effect of light irradiation on the gas-sensing properties of nanocomposites based on ZnO and Ag nanoparticles. Appl. Surf. Sci. 2020, 505, 144625. [Google Scholar] [CrossRef]
- Chinh, N.D.; Kim, C.; Kim, D. UV-light-activated H2S gas sensing by a TiO2 nanoparticulate thin film at room temperature. J. Alloys Compd. 2019, 778, 247–255. [Google Scholar] [CrossRef]
- Trawka, M.P.; Smulko, J.; Hasse, L.Z.; Granqvist, C.-G.; Ionescu, R.; Llobet, E.; Annanouch, F.E.; Kish, L.B. UV-Light Induced Fluctuation Enhanced Sensing by WO3-based Gas Sensors. IEEE Sens. J. 2016, 16, 5152–5159. [Google Scholar] [CrossRef]
- Trawka, M.; Smulko, J.; Hasse, L.; Granqvist, C.-G.; Annanouch, F.E.; Ionescu, R. Fluctuation enhanced gas sensing with WO3-based nanoparticle gas sensors modulated by UV light at selected wavelengths. Sens. Actuators B Chem. 2016, 234, 453–461. [Google Scholar] [CrossRef]
- Su, P.-G.; Yu, J.-H.; Pi-Guey, S.; Jia-Hao, Y. Enhanced NO2 gas-sensing properties of Au-Ag bimetal decorated MWCNTs/WO3 composite sensor under UV-LED irradiation. Sens. Actuators A Phys. 2020, 303, 111718. [Google Scholar] [CrossRef]
- Gonzalez, O.; Welearegay, T.G.; Llobet, E.; Vilanova, X. Pulsed UV Light Activated Gas Sensing in Tungsten Oxide Nanowires. Procedia Eng. 2016, 168, 351–354. [Google Scholar] [CrossRef]
- Saidi, T.; Palmowski, D.; Babicz-Kiewlicz, S.; Welearegay, T.G.; El Bari, N.; Ionescu, R.; Smulko, J.; Bouchikhi, B. Exhaled breath gas sensing using pristine and functionalized WO3 nanowire sensors enhanced by UV-light irradiation. Sens. Actuators B Chem. 2018, 273, 1719–1729. [Google Scholar] [CrossRef]
- Cho, M.; Park, I. Recent Trends of Light-enhanced Metal Oxide Gas Sensors: Review. J. Sens. Sci. Technol. 2016, 25, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Yin, M.; Yan, J.; Yang, N.; Liu, S. Controllable synthesis of Ag-WO3 core-shell nanospheres for light-enhanced gas sensors. Sens. Actuators B Chem. 2017, 251, 583–589. [Google Scholar] [CrossRef]
- Lemire, C.; Lollman, D.B.; Al Mohammad, A.; Gillet, E.; Aguir, K. Reactive R.F. magnetron sputtering deposition of WO3 thin films. Sens. Actuators B Chem. 2002, 84, 43–48. [Google Scholar] [CrossRef]
- Hu, L.; Hu, P.; Chen, Y.; Lin, Z.; Qiu, C. Synthesis and Gas-Sensing Property of Highly Self-assembled Tungsten Oxide Nanosheets. Front. Chem. 2018, 6, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Nakakura, S.; Arif, A.F.; Rinaldi, F.G.; Hirano, T.; Tanabe, E.; Balgis, R.; Ogi, T. Direct synthesis of highly crystalline single-phase hexagonal tungsten oxide nanorods by spray pyrolysis. Adv. Powder Technol. 2019, 30, 6–12. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Imamura, S.; Ito, S.; Nishio, K.; Fujimoto, K. Influence of oxygen gas concentration on hydrogen sensing of Pt/WO3 thin film prepared by sol–gel process. Sens. Actuators B Chem. 2015, 216, 394–401. [Google Scholar] [CrossRef]
- Chai, Y.; Ha, F.; Yam, F.; Hassan, Z. Fabrication of Tungsten Oxide Nanostructure by Sol-Gel Method. Procedia Chem. 2016, 19, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Poongodi, S.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Meena, P.; Masuda, Y.; Lee, C. Electrodeposition of WO3 nanostructured thin films for electrochromic and H2S gas sensor applications. J. Alloys Compd. 2017, 719, 71–81. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.; Liu, S.; Tan, M.; Cao, M. Electrodeposited tungsten oxide films onto porous silicon for NO2 detection at room temperature. J. Alloys Compd. 2018, 735, 718–727. [Google Scholar] [CrossRef]
- Zhou, D.; Che, B.; Kong, J.; Lu, X. A nanocrystalline tungsten oxide electrochromic coating with excellent cycling stability prepared via a complexation-assisted sol–gel method. J. Mater. Chem. C 2016, 4, 8041–8051. [Google Scholar] [CrossRef]
- Au, B.W.-C.; Chan, K.Y.; Knipp, D. Effect of film thickness on electrochromic performance of sol-gel deposited tungsten oxide (WO3). Opt. Mater. 2019, 94, 387–392. [Google Scholar] [CrossRef]
- Malakauskaite-Petruleviciene, M.; Stankeviciute, Z.; Niaura, G.; Prichodko, A.; Kareiva, A. Synthesis and characterization of sol–gel derived calcium hydroxyapatite thin films spin-coated on silicon substrate. Ceram. Int. 2015, 41, 7421–7428. [Google Scholar] [CrossRef]
- Hariharan, V.; Radhakrishnan, S.; Parthibavarman, M.; Dhilipkumar, R.; Chinnathambi, S. Synthesis of polyethylene glycol (PEG) assisted tungsten oxide (WO3) nanoparticles for l-dopa bio-sensing applications. Talanta 2011, 85, 2166–2174. [Google Scholar] [CrossRef]
- Ng, K.H.; Minggu, L.J.; Kassim, M.B. Gallium-doped tungsten trioxide thin film photoelectrodes for photoelectrochemical water splitting. Int. J. Hydrog. Energy 2013, 38, 9585–9591. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, J.; Bai, J.; Li, X.; Xia, L.; Zhou, B. Preparation of vertically aligned WO3 nanoplate array films based on peroxotungstate reduction reaction and their excellent photoelectrocatalytic performance. Appl. Catal. B Environ. 2017, 202, 388–396. [Google Scholar] [CrossRef]
- Amano, F.; Li, D.; Ohtani, B. Fabrication and photoelectrochemical property of tungsten(vi) oxide films with a flake-wall structure. Chem. Commun. 2010, 46, 2769–2771. [Google Scholar] [CrossRef] [Green Version]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, Z.; Guo, T.; Li, H.; Xue, Q. Synthesis of nanowire bundle-like WO3-W18O49 heterostructures for highly sensitive NH3 sensor application. J. Hazard. Mater. 2018, 353, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Lee, Y.B.; Choi, E.Y.; Park, H.C.; Park, S.S. Synthesis of nickel powders from various aqueous media through chemical reduction method. Mater. Chem. Phys. 2004, 86, 420–424. [Google Scholar] [CrossRef]
- Imran, M.; Rashid, S.S.A.A.H.; Sabri, Y.M.; Motta, N.; Tesfamichael, T.; Sonar, P.M.; Shafiei, M.; Rashi, S.S.A.A.H. Template based sintering of WO3 nanoparticles into porous tungsten oxide nanofibers for acetone sensing applications. J. Mater. Chem. C 2019, 7, 2961–2970. [Google Scholar] [CrossRef]
- Hatel, R.; Baitoul, M. Nanostructured Tungsten Trioxide (WO3): Synthesis, structural and morphological investigations. J. Phys. Conf. Ser. 2019, 1292, 012014. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, W.; Chen, X.; Zhang, B.; Wei, D.; Gao, S.; Cui, B. Nitrogen dioxide sensing using tungsten oxide microspheres with hierarchical nanorod-assembled architectures by a complexing surfactant-mediated hydrothermal route. J. Mater. Chem. A 2016, 4, 1345–1352. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Yang, Z.; Yu, Z.; Zhang, X.; Chang, T.C.; Javey, A. Vertically aligned tungsten oxide nanorod film with enhanced performance in photoluminescence humidity sensing. Sens. Actuators B Chem. 2014, 202, 708–713. [Google Scholar] [CrossRef]
- Mendoza-Agüero, N.; Agarwal, V. Optical and structural characterization of tungsten oxide electrodeposited on nanostructured porous silicon: Effect of annealing atmosphere and temperature. J. Alloys Compd. 2013, 581, 596–601. [Google Scholar] [CrossRef]
- Patil, V.; Adhyapak, P.; Suryavanshi, S.; Mulla, I. Oxalic acid induced hydrothermal synthesis of single crystalline tungsten oxide nanorods. J. Alloys Compd. 2014, 590, 283–288. [Google Scholar] [CrossRef]
- Wang, N.; Sun, J.; Cao, X.; Zhu, Y.; Wang, Q.; Wang, G.; Han, Y.; Lu, G.; Pang, G.; Feng, S. High-performance gas sensing achieved by mesoporous tungsten oxide mesocrystals with increased oxygen vacancies. J. Mater. Chem. A 2013, 1, 8653–8657. [Google Scholar] [CrossRef]
- Su, C.-Y.; Lin, H.-C. Direct Route to Tungsten Oxide Nanorod Bundles: Microstructures and Electro-Optical Properties. J. Phys. Chem. C 2009, 113, 4042–4046. [Google Scholar] [CrossRef]
- Feng, M.; Pan, A.L.; Zhang, H.; Li, Z.A.; Liu, F.; Liu, H.; Shi, D.X.; Zou, B.S.; Gao, H.J. Strong photoluminescence of nanostructured crystalline tungsten oxide thin films. Appl. Phys. Lett. 2005, 86, 141901. [Google Scholar] [CrossRef] [Green Version]
- Chernyakova, K.; Karpicz, R.; Zavadski, S.; Poklonskaya, O.; Jagminas, A.; Vrublevsky, I. Structural and fluorescence characterization of anodic alumina/carbon composites formed in tartaric acid solution. J. Lumin. 2017, 182, 233–239. [Google Scholar] [CrossRef]
- Chernyakova, K.; Karpicz, R.; Rutkauskas, D.; Vrublevsky, I.; Hassel, A.W. Structural and Fluorescence Studies of Polycrystalline α-Al2 O3 Obtained From Sulfuric Acid Anodic Alumina. Phys. Status Solidi (A) 2018, 215, 1700892. [Google Scholar] [CrossRef]
- Rückschloss, M.; Wirschem, T.; Tamura, H.; Rühl, G.; Oswald, J.; Veprek, S. Photoluminescence from OH-related radiative centres in silica, metal oxides and oxidized nanocrystalline and porous silicon. J. Lumin. 1995, 63, 279–287. [Google Scholar] [CrossRef]
- Chen, D.; Hou, X.; Li, T.; Yin, L.; Fan, B.; Wang, H.; Li, X.; Xu, H.; Lu, H.; Zhang, R.; et al. Effects of morphologies on acetone-sensing properties of tungsten trioxide nanocrystals. Sens. Actuators B Chem. 2011, 153, 373–381. [Google Scholar] [CrossRef]
- Shi, J.; Hu, G.; Sun, Y.; Geng, M.; Wu, J.; Liu, Y.; Ge, M.; Tao, J.; Cao, M.; Dai, N. WO3 nanocrystals: Synthesis and application in highly sensitive detection of acetone. Sens. Actuators B Chem. 2011, 156, 820–824. [Google Scholar] [CrossRef]
- Horsfall, L.A.; Pugh, D.C.; Blackman, C.; Parkin, I.P. An array of WO3 and CTO heterojunction semiconducting metal oxide gas sensors used as a tool for explosive detection. J. Mater. Chem. A 2017, 5, 2172–2179. [Google Scholar] [CrossRef]
- Vuong, N.M.; Kim, D.; Kim, H. Surface gas sensing kinetics of a WO3 nanowire sensor: Part 2—Reducing gases. Sens. Actuators B Chem. 2016, 224, 425–433. [Google Scholar] [CrossRef]
- Patil, V.B.; Adhyapak, P.V.; Patil, P.S.; Suryavanshi, S.S.; Mulla, I.S. Hydrothermally synthesized tungsten trioxide nanorods as NO2 gas sensors. Ceram. Int. 2015, 41, 3845–3852. [Google Scholar] [CrossRef]
- Ramanavičius, S.; Petrulevičienė, M.; Juodkazytė, J.; Grigucevičienė, A.; Ramanavicius, A. Selectivity of Tungsten Oxide Synthesized by Sol-Gel Method Towards Some Volatile Organic Compounds and Gaseous Materials in a Broad Range of Temperatures. Materials 2020, 13, 523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Yin, M.; Yan, J.; Liu, S. P-type sub-tungsten-oxide based urchin-like nanostructure for superior room temperature alcohol sensor. Appl. Surf. Sci. 2018, 441, 277–284. [Google Scholar] [CrossRef]
- Sayama, K. Production of High-Value-Added Chemicals on Oxide Semiconductor Photoanodes under Visible Light for Solar Chemical-Conversion Processes. ACS Energy Lett. 2018, 3, 1093–1101. [Google Scholar] [CrossRef]
- Cass, M.J.; Qiu, F.L.; Walker, A.B.; Fisher, A.C.; Peter, L.M. Influence of Grain Morphology on Electron Transport in Dye Sensitized Nanocrystalline Solar Cells. J. Phys. Chem. B 2003, 107, 113–119. [Google Scholar] [CrossRef]
- Amano, F.; Ishinaga, E.; Yamakata, A. Effect of Particle Size on the Photocatalytic Activity of WO3 Particles for Water Oxidation. J. Phys. Chem. C 2013, 117, 22584–22590. [Google Scholar] [CrossRef]
- Thimsen, E.; Rastgar, N.; Biswas, P. Nanostructured TiO2 Films with Controlled Morphology Synthesized in a Single Step Process: Performance of Dye-Sensitized Solar Cells and Photo Watersplitting. J. Phys. Chem. C 2008, 112, 4134–4140. [Google Scholar] [CrossRef]






| Sample | Coating Formation Time (min) | Crystallite Size (nm) |
|---|---|---|
| PrOH-140 | 140 | 6.57 |
| PrOH-180 | 180 | 5.58 |
| EtOH-140 | 140 | 5.51 |
| EtOH-180 | 180 | 5.55 |
| Sample | λem, nm | τ1, ns (%) | τ2, ns (%) | τ3, ns (%) | τave, ns |
|---|---|---|---|---|---|
| PrOH-140 | 426 | 1.6 (37%) | 4.1 (32%) | 30 (31%) | 11.2 |
| PrOH-180 | 428 | 1.6 (37%) | 4.4 (32%) | 30 (31%) | 11.3 |
| EtOH-140 | 436 | 0.7 (55%) | 2.6 (30%) | 22 (15%) | 4.5 |
| EtOH-180 | 443 | 1.5 (33%) | 3.9 (36%) | 29 (31%) | 10.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruleviciene, M.; Juodkazyte, J.; Parvin, M.; Tereshchenko, A.; Ramanavicius, S.; Karpicz, R.; Samukaite-Bubniene, U.; Ramanavicius, A. Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions. Materials 2020, 13, 2814. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13122814
Petruleviciene M, Juodkazyte J, Parvin M, Tereshchenko A, Ramanavicius S, Karpicz R, Samukaite-Bubniene U, Ramanavicius A. Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions. Materials. 2020; 13(12):2814. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13122814
Chicago/Turabian StylePetruleviciene, Milda, Jurga Juodkazyte, Maliha Parvin, Alla Tereshchenko, Simonas Ramanavicius, Renata Karpicz, Urte Samukaite-Bubniene, and Arunas Ramanavicius. 2020. "Tuning the Photo-Luminescence Properties of WO3 Layers by the Adjustment of Layer Formation Conditions" Materials 13, no. 12: 2814. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13122814