Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Structural Characterization
2.4. Compositional Analysis
2.5. In Vitro Evaluation of Remineralization and Dentinal Tubules Occlusion
2.6. Statistical Analysis
3. Results and Discussion
3.1. Structural Characterization
3.2. Compositional Analysis
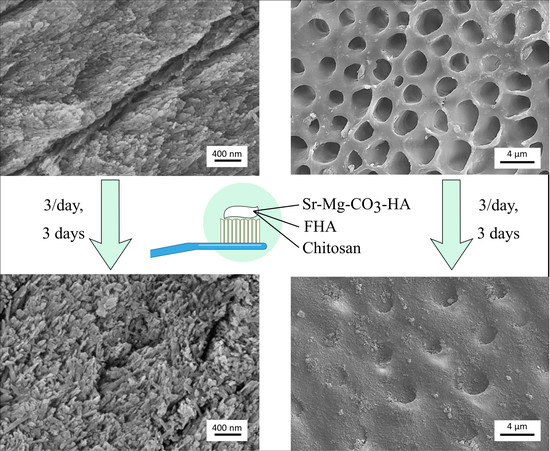
3.3. In Vitro Evaluation of Remineralization
3.4. In Vitro Evaluation of Dentinal Tubules Occlusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neel, E.A.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef]
- Enax, J.; Epple, M. Synthetic hydroxyapatite as a biomimetic oral care agent. Oral Health Prev. Dent. 2018, 16, 7–19. [Google Scholar] [PubMed]
- Roveri, N.; Battistella, E.; Bianchi, C.L.; Foltran, I.; Foresti, E.; Iafisco, M.; Lelli, M.; Naldoni, A.; Palazzo, B.; Rimondini, L. Surface enamel remineralization: Biomimetic apatite nanocrystals and fluoride ions different effects. J. Nanomater. 2009. [Google Scholar] [CrossRef] [Green Version]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, X.; Wu, Y. Remineralizing Nanomaterials for Minimally Invasive Dentistry. In Nanotechnology in Endodontics: Current and Potential Clinical Applications; Kishen, A., Ed.; Springer International Publishing: Cham, Germany, 2015; pp. 173–193. [Google Scholar]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Dental plaque as a biofilm and a microbial community—Implications for treatment. J. Oral Biosci. 2015, 57, 185–191. [Google Scholar] [CrossRef]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Ecological approaches to oral biofilms: Control without killing. Caries Res. 2015, 49, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Zero, D.T. Dentifrices, mouthwashes, and remineralization/caries arrestment strategies. BMC Oral Health 2006, 6, S9. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Beltrami, R.; Rattalino, D.; Mirando, M.; Chiesa, M.; Poggio, C. Protective effects of a zinc-hydroxyapatite toothpaste on enamel erosion: SEM study. Ann. Stomatol. 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Lynch, R.; Navada, R.; Walia, R. Low-levels of fluoride in plaque and saliva and their effects on the demineralisation and remineralisation of enamel; role of fluoride toothpastes. Int. Dent. J. 2004, 54, 304–309. [Google Scholar] [CrossRef]
- Ten Cate, J.M. Contemporary perspective on the use of fluoride products in caries prevention. Br. Dent. J. 2013, 214, 161–167. [Google Scholar] [CrossRef] [PubMed]
- DenBesten, P.; Li, W. Chronic fluoride toxicity: Dental fluorosis. In Fluoride and the Oral Environment; Karger Publishers: Basel, Switzerland, 2011; Volume 22, pp. 81–96. [Google Scholar]
- Arnaud, T.M.S.; de Barros Neto, B.; Diniz, F.B. Chitosan effect on dental enamel de-remineralization: An in vitro evaluation. J. Dent. 2010, 38, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Ohara, N.; Ganno, T.; Yamaguchi, K.; Ishizaki, T.; Nakamura, T.; Sato, M. Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch. Oral Biol. 2007, 52, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-S.; Tsai, S.; Kuo, C.-C.; Bassani, A.W.; Pepe-Mooney, B.; Miksa, D.; Masters, J.; Sullivan, R.; Composto, R.J. Chitosan adsorption on hydroxyapatite and its role in preventing acid erosion. J. Colloid Interface Sci. 2012, 385, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan use in dentistry: A systematic review of recent clinical studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, A.C.; Cazzaniga, G.; Ottobelli, M.; Garcia-Godoy, F.; Brambilla, E. Substituted Nano-Hydroxyapatite toothpastes reduce biofilm formation on enamel and resin-based composite surfaces. J. Funct. Biomater. 2020, 11, 36. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Yu, H. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed. Mater. 2009, 4, 034104. [Google Scholar] [CrossRef] [Green Version]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Lelli, M.; Putignano, A.; Marchetti, M.; Foltran, I.; Mangani, F.; Procaccini, M.; Roveri, N.; Orsini, G. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: A comparative in vivo study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roveri, N.; Battistella, E.; Foltran, I.; Foresti, E.; Iafisco, M.; Lelli, M.; Palazzo, B.; Rimondini, L. Synthetic biomimetic carbonate-hydroxyapatite nanocrystals for enamel remineralization. Adv. Mater. Res. 2008, 47, 821–824. [Google Scholar] [CrossRef]
- Berg, C.; Unosson, E.; Riekehr, L.; Xia, W.; Engqvist, H. Electron microscopy evaluation of mineralization on peritubular dentin with amorphous calcium magnesium phosphate microspheres. Ceram. Int. 2020, 46, 19469–19475. [Google Scholar] [CrossRef]
- Steinert, S.; Zwanzig, K.; Doenges, H.; Kuchenbecker, J.; Meyer, F.; Enax, J. Daily Application of a Toothpaste with Biomimetic Hydroxyapatite and Its Subjective Impact on Dentin Hypersensitivity, Tooth Smoothness, Tooth Whitening, Gum Bleeding, and Feeling of Freshness. Biomimetics 2020, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, I.C.; Rezende, C.C.; da Silva, J.A.F.; de Jesus, D.P. Simultaneous determination of free fluoride and monofluorophosphate in toothpaste by capillary electrophoresis with capacitively coupled contactless conductivity detection. Talanta 2009, 78, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Que, K.; Wang, H.; An, R.; Chen, Z.; Qiu, Z.; Lin, M.; Song, J.; Yang, J.; Lu, D. Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimaeric peptides. Dent. Mater. 2017, 33, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Casado, F.J.; Iafisco, M.; Delgado-López, J.M.; Martínez-Benito, C.; Ruiz-Pérez, C.; Colangelo, D.; Oltolina, F.; Prat, M.; Gómez-Morales, J. Bioinspired Citrate–Apatite Nanocrystals Doped with Divalent Transition Metal Ions. Cryst. Growth Des. 2015, 16, 145–153. [Google Scholar] [CrossRef]
- Delgado-López, J.M.; Iafisco, M.; Rodríguez, I.; Tampieri, A.; Prat, M.; Gómez-Morales, J. Crystallization of bioinspired citrate-functionalized nanoapatite with tailored carbonate content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef]
- Iafisco, M.; Ramirez-Rodriguez, G.B.; Sakhno, Y.; Tampieri, A.; Martra, G.; Gomez-Morales, J.; Delgado-Lopez, J.M. The growth mechanism of apatite nanocrystals assisted by citrate: Relevance to bone biomineralization. CrystEngComm 2015, 17, 507–511. [Google Scholar] [CrossRef]
- Saravanapavan, P.; Hench, L.L. Mesoporous calcium silicate glasses. I. Synthesis. J. Non-Cryst. Solids 2003, 318, 1–13. [Google Scholar] [CrossRef]
- Roche, K.J.; Stanton, K.T. Measurement of fluoride substitution in precipitated fluorhydroxyapatite nanoparticles. J. Fluor. Chem. 2014, 161, 102–109. [Google Scholar] [CrossRef]
- Iafisco, M.; Ruffini, A.; Adamiano, A.; Sprio, S.; Tampieri, A. Biomimetic magnesium–carbonate-apatite nanocrystals endowed with strontium ions as anti-osteoporotic trigger. Mater. Sci. Eng. C 2014, 35, 212–219. [Google Scholar] [CrossRef]
- Anderson, D.; Smith, A.L. Analysis of Silicones; Wiley-Interscience: New York, NY, USA, 1974. [Google Scholar]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Abdullah, O.; Badshah, S.F. Preparation and Evaluation of Skin Wound Healing Chitosan-Based Hydrogel Membranes. AAPS PharmSciTech 2018, 19, 3199–3209. [Google Scholar] [CrossRef]
- Robinson, C.; Weatherell, J.; Hallsworth, A. Variation in composition of dental enamel within thin ground tooth sections. Caries Res. 1971, 5, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Angmar, B.; Carlström, D.; Glas, J.-E. Studies on the ultrastructure of dental enamel: IV. The mineralization of normal human enamel. J. Ultrastruct. Res. 1963, 8, 12–23. [Google Scholar] [CrossRef]
- Yoshiyama, M.; Masada, J.; Uchida, A.; Ishida, H. Scanning electron microscopic characterization of sensitive vs. insensitive human radicular dentin. J. Dent. Res. 1989, 68, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chiba, A.; Scheffel, D.L.; Hebling, J.; Agee, K.; Niu, L.-N.; Tay, F.R.; Pashley, D.H. Effects of a dicalcium and tetracalcium phosphate-based desensitizer on in vitro dentin permeability. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]






| Commercial Name | Ingredients |
|---|---|
| Biosmalto Caries Abrasion and Erosion | Purified water, glycerin, hydrated silica, fluorohydroxyapatite, magnesium-strontium-carbonate-hydroxyapatite conjugated with chitosan, cellulose gum, xylitol, cocamidopropyl betaine, xantham gum, aroma, sodium monofluorophosphate, potassium acesulfame, ethylhexylglicerin, phenoxyethanol, sodium benzoate, citric acid. |
| Sample | Si (wt%) a | Ca (wt%) a | P (wt%) a | Mg (wt%) a | Sr (wt%) a | F (ppm) b | Ca/P (mol) a |
|---|---|---|---|---|---|---|---|
| Whole toothpaste | 3.5 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | 0.02 ± 0.01 | 0.09 ± 0.01 | 1458 ± 25 | 0.96 ± 0.05 |
| Water-insoluble fraction | 29.8 ± 1.9 | 9.2 ± 0.3 | 4.6 ± 0.2 | 0.20 ± 0.01 | 0.72 ± 0.01 | 1390 ± 25 | 1.51 ± 0.01 |
| Sample | Si (wt%) | Ca (wt%) | P (wt%) | Mg (wt%) | Sr (wt%) | F (wt%) | Ca/P (mol) |
|---|---|---|---|---|---|---|---|
| Enamel, control | 0.07 ± 0.01 | 37.9 ± 0.1 | 16.4 ± 0.1 | 0.3 ± 0.1 | - | - | 1.79 ± 0.01 |
| Enamel, treated | 1.2 ± 0.1 | 35.4 ± 0.1 | 14.5 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.22 ± 0.05 | 1.89 ± 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degli Esposti, L.; Ionescu, A.C.; Brambilla, E.; Tampieri, A.; Iafisco, M. Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules. Materials 2020, 13, 2928. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13132928
Degli Esposti L, Ionescu AC, Brambilla E, Tampieri A, Iafisco M. Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules. Materials. 2020; 13(13):2928. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13132928
Chicago/Turabian StyleDegli Esposti, Lorenzo, Andrei C. Ionescu, Eugenio Brambilla, Anna Tampieri, and Michele Iafisco. 2020. "Characterization of a Toothpaste Containing Bioactive Hydroxyapatites and In Vitro Evaluation of Its Efficacy to Remineralize Enamel and to Occlude Dentinal Tubules" Materials 13, no. 13: 2928. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13132928