Effect of Blast-Furnace Slag Replacement Ratio and Curing Method on Pore Structure Change after Carbonation on Cement Paste
Abstract
:1. Introduction
2. Experimental Program
2.1. Experimental Materials and Sample Preparation
2.2. Experimental Method
2.2.1. Mercury Intrusion Porosimetry
2.2.2. X-ray Diffraction
2.2.3. Thermal Analysis
2.3. Curing Condition
3. Experiment Result
3.1. Characteristics of Chemical Properties According to Carbonation
X-ray Diffraction Result
3.2. Characteristics of the Amount of Calcium Hydroxide and Calcium Carbonate after Carbonation
3.2.1. DTG Curves of Calcium Hydroxide and Calcium Carbonate
3.2.2. The Amount of Calcium Hydroxide and Calcium Carbonate
3.2.3. Carbonation of CH and C–S–H
3.3. Characteristics of Pore Structure after Carbonation
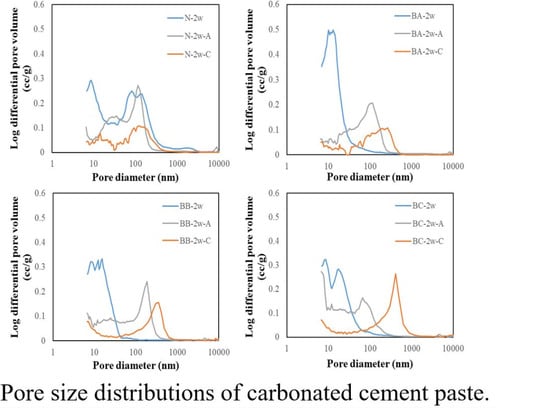
3.3.1. Pore Volume of Log Differential Results
3.3.2. Cumulative Pore Volume Results
3.3.3. Total Porosity Results
4. Discussion of Pores Affecting Durability
5. Conclusions
- The small pore distribution tends to increase as the BFS replacement ratio increases during water curing. Moreover, it was found that the carbonation of the cementitious composite could enhance the density of the cement paste samples. A shift towards a large pore distribution is caused after carbonation.
- With respect to the influence of the DTG curve on each curing environment, it is notable that as the BFS replacement rate increased, the peak of the produced CC tended to decrease.
- With respect to the influence of the amount of CH and C–S–H on carbonation, the thermal analysis of the carbonation amount from C–S–H increased with the BFS replacement ratios of the BB specimen and the BC specimen in the carbonation samples.
- With respect to the influence of the cement mineral composite on the carbonated samples, it is notable that the amount of vaterite in the BFS-blended paste samples is larger than that of the N samples, and this phenomenon is primarily due to the low Ca/Si ratio of the C–S–H phase caused by BFS replacement.
Author Contributions
Funding
Conflicts of Interest
References
- Tanaka, Y.; Saeki, T.; Sasaki, K.; Suda, Y. Fundamental Study on Density of C-S-H. Cem. Sci. Concr. Technol. 2009, 63, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Ito, Y.; Kawai, K. Carbonation Characteristics of The C-S-H With Various Ca/Si Ratios. Cem. Sci. Concr. Technol. 2013, 67, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Ishida, S.; Igarashi, S.-I.; Koike, Y. Effects of Early-Age Carbonation on Mecanical and Electrical Properties of Cement Pastes and Concretes. Cem. Sci. Concr. Technol. 2011, 65, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Saeki, T.; Ohga, H.; Nagataki, S. Change in micro-structure of concrete due to carbonation. Doboku Gakkai Ronbunshu 1990, 420, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-H.; Nakarai, K.; Ishii, Y.; Yokozuka, K. Experimental Study on Effect of Carbonation of Early Aged Cement Paste on Micro-Pore Structure and Effective Diffusion Coefficient Of Oxygen. Cem. Sci. Concr. Technol. 2009, 63, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Phung, Q.T.; Maes, N.; Jacques, D.; Bruneel, E.; Van Driessche, I.; Ye, G.; De Schutter, G. Effect of limestone fillers on microstructure and permeability due to carbonation of cement pastes under controlled CO2 pressure conditions. Constr. Build. Mater. 2015, 82, 376–390. [Google Scholar] [CrossRef]
- Borges, P.H.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C–S–H in composite cement pastes containing high amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J.; He, Z. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Na, S.H.; Kim, J.H.; Choi, H.G.; Hama, Y. Evaluation of the combined deterioration by freeze–thaw and carbonation of mortar incorporating BFS, limestone powder and calcium sulfate. Mater. Struct. 2017, 50, 171. [Google Scholar] [CrossRef]
- Zhang, W.; Zakaria, M.; Hama, Y. Influence of aggregate materials characteristics on the drying shrinkage properties of mortar and concrete. Constr. Build. Mater. 2013, 49, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Hama, Y.; Na, S.H. Drying shrinkage and microstructure characteristics of mortar incorporating ground granulated blast furnace slag and shrinkage reducing admixture. Constr. Build. Mater. 2015, 93, 267–277. [Google Scholar] [CrossRef]
- Kayyali, O.A. Mercury intrusion porosimetry of concrete aggregates. Mater. Struct. 1985, 18, 259–262. [Google Scholar] [CrossRef]
- Karen, S.; Ruben, S.; Barbara, L.I. A Practical Guide to Microstructural Analysis of Cementitious Materials; Taylor and Francis Group: Oxford, UK, 2015. [Google Scholar]
- Mo, L.; Hao, Y.; Liu, Y.; Wang, F.; Deng, M. Preparation of calcium carbonate binders via CO2 activation of magnesium slag. Cem. Concr. Res. 2019, 121, 81–90. [Google Scholar] [CrossRef]
- Wu, B.; Ye, G. Development of porosity of cement paste blended with supplementary cementitious materials after carbonation. Constr. Build. Mater. 2017, 145, 52–61. [Google Scholar] [CrossRef]
- Thiéry, M.; Faure, P.; Morandeau, A.; Platret, G.; Bouteloup, J.-F.; Dangla, P. Effect of carbonation on the microstructure and moisture properties of cement-based materials. In Proceedings of the XII DBMC 12th International Conference on Building Materials and Components, Porto, Portugal, 12–15 April 2011. [Google Scholar]
- Jin, S.; Zheng, G.; Yu, J. A micro freeze-thaw damage model of concrete with fractal dimension. Constr. Build. Mater. 2020, 257, 119434. [Google Scholar] [CrossRef]
- Li, B.; Mao, J.; Nawa, T.; Han, T. Mesoscopic damage model of concrete subjected to freeze-thaw cycles using mercury intrusion porosimetry and differential scanning calorimetry (MIP-DSC). Constr. Build. Mater. 2017, 147, 79–90. [Google Scholar] [CrossRef]
- Vu, T.H.; Gowripalan, N.; De Silva, P.; Paradowska, A.Z.; Garbe, U.; Kidd, P.; Sirivivatnanon, V. Assessing carbonation in one-part fly ash/slag geopolymer mortar: Change in pore characteristics using the state-of-the-art technique neutron tomography. Cem. Concr. Compos. 2020, 114, 103759. [Google Scholar] [CrossRef]
- Vineet, S.; Karen, S.; Bishwajit, B.; Shashank, B. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Zhang, W.; Zakaria, M.; Kishimoto, Y.; Hama, Y. Drying Shrinkage and Microstructure Characteristics of Ground Granulated Blast Furnace Slag-Cement Mortar; JCI Annual Convention: Hirosima, Japan, July 2012. [Google Scholar]
- Çetin, C.; Erdoğan, S.T.; Tokyay, M. Effect of particle size and slag content on the early hydration of interground blended cements. Cem. Concr. Compos. 2016, 67, 39–49. [Google Scholar] [CrossRef]
- Barnett, S.; Soutsos, M.; Millard, S.; Bungey, J. Strength development of mortars containing ground granulated blast-furnace slag: Effect of curing temperature and determination of apparent activation energies. Cem. Concr. Res. 2006, 36, 434–440. [Google Scholar] [CrossRef]
- Boukendakdji, O.; Kadri, E.-H.; Kenai, S. Effects of granulated blast furnace slag and superplasticizer type on the fresh properties and compressive strength of self-compacting concrete. Cem. Concr. Compos. 2012, 34, 583–590. [Google Scholar] [CrossRef]
- Miyazawa, S.; Yokomuro, T.; Sakai, E.; Yatagai, A.; Nito, N.; Koibuchi, K. Properties of concrete using high C3S cement with ground granulated blast-furnace slag. Constr. Build. Mater. 2014, 61, 90–96. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.I. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Prithika, A.J.; Sekar, S. Mechanical and fracture characteristics of Eco-friendly concrete produced using coconut shell, ground granulated blast furnace slag and manufactured sand. Constr. Build. Mater. 2016, 103, 1–7. [Google Scholar] [CrossRef]
- Yoshida, S.; Nawa, T.; Taguchi, F.; Watanabe, H. Influence of Pore Structure on Carbonation of Belite-Based Cement Concrete Using Ground Granulated Blast-Furnace Slag at Low Water/Binder Ratio. Doboku Gakkai Ronbunshuu E 2008, 64, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Moro, F.; Böhni, H. Ink-Bottle Effect in Mercury Intrusion Porosimetry of Cement-Based Materials. J. Colloid Interface Sci. 2002, 246, 135–149. [Google Scholar] [CrossRef]
- Deprez, M.; De Kock, T.; De Schutter, G.; Cnudde, V. The role of ink-bottle pores in freeze-thaw damage of oolithic limestone. Constr. Build. Mater. 2020, 246, 118515. [Google Scholar] [CrossRef]
- Prick, A. Dilatometrical behaviour of porous calcareous rock samples subjected to freeze-thaw cycles. Catena 1995, 25, 7–20. [Google Scholar] [CrossRef]
- Shibuya, M.; Atarashi, D.; Hama, Y.; Ueda, N.; Aono, Y. Influence of Curing Condition and Classification of Cement to Change of Pore Structure and Frost Resistance of Mortal. AIJ 2009, 82, 5–10. [Google Scholar]
- Aono, Y.; Fumiaki, M.; Sumio, S.; Hama, Y. Influence of Nanostructure Change during Drying on Frost Resistance of HCP. Concr. Res. Technol. 2008, 19, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Ghantous, R.M.; Farnam, Y.; Unal, E.; Weiss, J.; Weiss, W.J. The influence of carbonation on the formation of calcium oxychloride. Cem. Concr. Compos. 2016, 73, 185–191. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]















| Chemical Composition (%) | Physical Properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | LOI | Density (g/cm3) | Blaine Fineness (cm2/g) | |
| OPC | 21.06 | 5.51 | 2.69 | 65.47 | 1.66 | 0.4 | 0.24 | 1.91 | 2.28 | 3.17 | 3390 |
| BFS | 34.03 | 14.36 | 0.83 | 43.28 | 6.51 | - | - | - | 0.1 | 2.91 | 3930 |
| W/C | Replacement Ratio (%) | ||
|---|---|---|---|
| OPC | BFS | ||
| N | 0.65 | 100 | - |
| BA | 85 | 15 | |
| BB | 55 | 45 | |
| BC | 35 | 65 | |
| Water Curing | Air Curing | Carbonation | |
|---|---|---|---|
| N | 0.37 | 0.29 | 0.37 |
| BA | 0.37 | 0.32 | 0.37 |
| BB | 0.36 | 0.32 | 0.37 |
| BC | 0.36 | 0.32 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Na, S.; Hama, Y. Effect of Blast-Furnace Slag Replacement Ratio and Curing Method on Pore Structure Change after Carbonation on Cement Paste. Materials 2020, 13, 4787. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214787
Kim J, Na S, Hama Y. Effect of Blast-Furnace Slag Replacement Ratio and Curing Method on Pore Structure Change after Carbonation on Cement Paste. Materials. 2020; 13(21):4787. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214787
Chicago/Turabian StyleKim, Junho, Seunghyun Na, and Yukio Hama. 2020. "Effect of Blast-Furnace Slag Replacement Ratio and Curing Method on Pore Structure Change after Carbonation on Cement Paste" Materials 13, no. 21: 4787. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214787