Removal of Heavy Metal Ions from Wastewaters: An Application of Sodium Trithiocarbonate and Wastewater Toxicity Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Synthesis of Sodium Trithiocarbonate (Na2CS3)
2.2. Origin and Physicochemical Parameters of Galvanic Wastewater
2.3. Apparatus and Experiment Conditions
2.4. Analytical Procedures
2.5. Optimization of the Experiments
3. Results and Discussion
3.1. Physicochemical Parameters of Synthesized Na2CS3 Solution
3.2. Physicochemical Parameters of Galvanic Wastewater
3.3. CCD/RSM Results
3.4. Toxicity Assessment
4. Conclusions
- The use of Na2CS3 under optimal conditions determined with the use of CCD/RSM enables effective precipitation of heavy metals such as Cu, Cd and Zn from actual galvanic wastewater, even in the presence of complexing compounds.
- The conventional methods of metal precipitation used so far, which consist of the alkalization of wastewater to the appropriate pH value in order to precipitate metal hydroxides, are less effective compared to the proposed methodology.
- The use of rotifer B. plicatilis to assess the toxicity of treated wastewater indicated that it has decreased significantly, which is beneficial from an environmental point of view.
- The application of Na2CS3 is not complicated and can be easily used in metal surface treatment plants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadirvelu, K.; Thamaraiselvi, K.; Namasivayam, C. Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresour. Technol. 2001, 76, 63–65. [Google Scholar] [CrossRef]
- Williams, C.J.; Aderhold, D.; Edyvean, G.J. Comparison between biosorbents for the removal of metal ions from aqueous solutions. Water Res. 1998, 32, 216–224. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Jaishankar, M.; Mathew, B.B.; Shah, M.S.; Gowda, K.R.S. Biosorption of Few Heavy Metal Ions Using Agricultural Wastes. J. Environ. Pollut. Hum. 2014, 2, 1–6. [Google Scholar]
- Wang, S.; Shi, X. Molecular mechanisms of metal toxicity and carcinogenesis. Mol. Cell. Biochem. 2001, 222, 3–9. [Google Scholar] [CrossRef]
- Chang, L.W.; Magos, L.; Suzuki, T. Toxicology of Metals; CRC Press: London, UK, 1996. [Google Scholar]
- Davidson, T.; Ke, Q.; Costa, M. Selected Molecular Mechanisms of Metal Toxicity and Carcinogenicity. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G., Fowler, B., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; Chapter 9; pp. 173–196. [Google Scholar]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol. 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Yedjou, G.C.; Tchounwou, P.B. In vitro cytotoxic and genotoxic effects of arsenic trioxide on human leukemia cells using the MTT and alkaline single cell gel electrophoresis (comet) assays. Mol. Cell. Biochem. 2007, 301, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Ishaque, A.; Schneider, J. Cytotoxicity and transcriptional activation of stress genes in human liver carcinoma cells (HepG2) exposed to cadmium chloride. Mol. Cell. Biochem. 2001, 222, 21–28. [Google Scholar] [CrossRef]
- Patlolla, A.; Barnes, C.; Field, J.; Hackett, D.; Tchounwou, P.B. Potassium dichromate-induced cytotoxicity, genotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int. J. Environ. Res. Public Health 2009, 6, 643–653. [Google Scholar] [CrossRef]
- Yedjou, G.C.; Tchounwou, P.B. N-acetyl-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int. J. Environ. Res. Public Health 2008, 4, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, D.J.; Tchounwou, P.B. Mercury induces the externalization of phosphatidylserine in human proximal tubule (HK-2) cells. Int. J. Environ. Res. Public Health 2007, 4, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudevan, S.; Oturan, M.A. Electrochemistry: As cause and cure in water pollution–An overview. Environ. Chem. Lett. 2014, 12, 97–108. [Google Scholar] [CrossRef]
- Salles, F.J.; Sato, A.P.S.; Luz, M.S.; Fávaro, D.I.T.; Ferreira, F.J.; da Silva Paganini, W.; Olympio, K.P.K. The environmental impact of informal and home productive arrangement in the jewelry and fashion jewelry chain on sanitary sewer system. Environ. Sci. Pollut. Res. 2018, 25, 10701–10713. [Google Scholar] [CrossRef] [PubMed]
- Giurlani, W.; Zangari, G.; Gambinossi, F.; Passaponti, M.; Salvietti, E.; Di Benedetto, F.; Caporali, S.; Innocenti, M. Electroplating for Decorative Applications: Recent Trends in Research and development. Coatings 2018, 8, 260. [Google Scholar] [CrossRef] [Green Version]
- Rahman, L.; Sarkar, S.M.; Yousef, M.M. Efficient removal of heavy metals from electroplating wastewater using polymer ligands. Front. Environ. Sci. Eng. 2016, 10, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Thomas, M.; Białecka, B.; Zdebik, D. Sources of copper ions and selected methods of their removal from wastewater from the printed circuits board production. Inż. Ekol. 2014, 37, 31–49. [Google Scholar]
- Thomas, M.; Białecka, B.; Zdebik, D. Evaluation and application of coagulants containing divalent and trivalent iron to enhance removal of organic compounds and complexed copper and tin ions from industrial effluents. Inż. Ekol. 2014, 38, 167–180. [Google Scholar]
- Głodniok, M.; Zdebik, D.; Thomas, M.; Zawartka, P. A toxic effect of wastewater from the production of printed circuit boards on activated sludge from municipal wastewater treatment plant. Przem. Chem. 2016, 95, 1304–1309. [Google Scholar]
- Thomas, M.; Zdebik, D.; Świnder, H. Recovery of tin from electroplating sludges generated in the treatment of the concentrated wastewater from electrochemical tin plating. Przem. Chem. 2017, 96, 1296–1302. [Google Scholar]
- Huisman, J.L.; Schouten, G.; Schultz, C. Biologically produced sulphide for purification of process streams, effluent treatment and recovery of metals in the metal and mining industry. Hydrometallurgy 2006, 83, 106–113. [Google Scholar] [CrossRef]
- Özverdi, A.; Erdem, M. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard. Mater. 2006, 137, 626–632. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, M.J.; Rodríguez, M.A.; Luquea, S.; Álvareza, J.R. Recovery of heavy metals from metal industry waste waters by chemical precipitation and nanofiltration. Desalination 2006, 200, 742–744. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Fatta, D.; Parperis, K.; Mentzis, A.; Haralambous, K.J.; Loizidou, M. Nickel uptake from a wastewater stream produced in a metal finishing industry by combination of ion-exchange and precipitation methods. Sep. Purif. Technol. 2004, 39, 181–188. [Google Scholar] [CrossRef]
- Jusoh, A.; Shiung, L.S.; Ali, N.; Noor, M.J.M.M. A simulation study of the removal efficiency of granular activated carbon on cadmium and lead. Desalination 2007, 206, 9–16. [Google Scholar] [CrossRef]
- Reyes, I.; Villarroel, M.; Diez, M.C.; Navia, R. Using lignimerin (a recovered organic material from Kraft cellulose mill wastewater) as sorbent for Cu and Zn retention from aqueous solutions. Bioresour. Technol. 2009, 100, 4676–4682. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.D.; Wang, S.W.; Hua, J.; Lu, Y.; Li, J.X.; Dong, Y.H.; Wang, X.K. Adsorption of Pb(II) on diatomite as affected via aqueous solution chemistry and temperature. Colloid Surf. 2009, 339, 159–166. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Sorption of Cu2+, Cd2+ and Pb2+ using modified zeolite from coal fly ash. Chem. Eng. J. 2008, 144, 245–258. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Batch and column studies of biosorption of heavy metals by Caulerpa lentillifera. Bioresour. Technol. 2008, 99, 2766–2777. [Google Scholar] [CrossRef]
- Landaburu-Aguirre, J.; García, V.; Pongrácz, E.; Keiski, R.L. The removal of zinc from synthetic wastewaters by micellar-enhanced ultrafiltration: Statistical design of experiments. Desalination 2009, 240, 262–269. [Google Scholar] [CrossRef]
- Molinari, R.; Poerio, T.; Argurio, P. Selective separation of copper (II) and nickel (II) from aqueous media using the complexation-ultrafiltration process. Chemosphere 2008, 70, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Mohsen-Nia, M.; Montazeri, P.; Modarress, H. Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 2007, 217, 276–281. [Google Scholar] [CrossRef]
- Figoli, A.; Cassano, A.; Criscuoli, A.; Mozumder, M.S.I.; Uddin, M.T.; Islam, M.A.; Drioli, E. Influence of operating parameters on the arsenic removal by nanofiltration. Water Res. 2010, 44, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kabdasli, I.; Arslan, T.; Olmez-Hanci, T.; Arslan-Alaton, I.; Tünay, O. Complexing agent and heavy metal removals from metal plating effluent by electrocoagulation with stainless steel electrodes. J. Hazard. Mater. 2009, 165, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Białecka, B.; Zdebik, D. Removal of copper, nickel and tin from model and real industrial wastewater using sodium trithiocarbonate. The negative impact of complexing compounds. Arch. Environ. Prot. 2018, 44, 33–47. [Google Scholar]
- Thomas, M.; Zdebik, D.; Białecka, B. Use of sodium trithiocarbonate for remove of chelated copper ions from industrial wastewater originating from the electroless copper plating process. Arch. Environ. Prot. 2018, 44, 32–42. [Google Scholar]
- Thomas, M.; Zdebik, D.; Białecka, B. Using sodium trithiocarbonate to precipitate heavy metals from industrial wastewater—From the laboratory to industrial scale. Pol. J. Environ. Stud. 2018, 27, 1–11. [Google Scholar] [CrossRef]
- Thomas, M.; Białecka, B.; Cykowska, M.; Bebek, M.; Bauerek, A. Precipitation of rare earth elements from model and real solutions by using alkaline and sulfur compounds. Przem. Chem. 2017, 96, 2471–2475. [Google Scholar]
- Elfline, G.S. Method of Removing Heavy Metal from Wastewater Streams. U.S. Patent 4,678,584, 7 July 1987. [Google Scholar]
- American Society for Testing Materials. Standard Practice for the Preparation of Substitute Ocean Water; ASTM D1141–98; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Supniewski, J. Inorganic Preparation; PWN Warsaw: Paris, France, 1985. [Google Scholar]
- Stechman, M.; Różycka, D.; Mateńko, H.; Marszałek, J.; Wojtycha, Z.; Jamroży, J.; Zaczkowski, L.; Sufczyński, T. Sodium Trithiocarbonate Manufacturing Method. PL 198,453, 1 November 2001. [Google Scholar]
- Gattow, G.; Behrendt, W. Carbon Sulfides and Their Inorganic and Complex Chemistry; Georg Thieme Publishers: Stuttgart, Germany, 1977. [Google Scholar]
- Bobrowska-Krajewska, K.; Dąbek, M.; Kmieć, B.; Krajewski, J. Possibility of removing a trace amounts of heavy metals from wastewater. Arch. Environ. Prot. 1994, 3–4, 73–87. [Google Scholar]
- Bobrowska-Krajewska, K.; Dąbek, M.; Kmieć, B.; Krajewski, J. Selected issues of synthesis and application of sodium triocarbonate. Chemik 1994, 6, 155–158. [Google Scholar]
- Polish Committee for Standardization. Sodium Sulphide for Industrial Use; PN-C-84042; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- International Organization for Standardization. Water Quality-Determination of pH; ISO 10523; IOS: Geneva, Switzerland, 2008. [Google Scholar]
- NANOCOLOR® Organische Komplexbildner 10. Available online: https://www.mn-net.com/media/pdf/6a/60/8c/Instruction-985052-Tube-test-NANOCOLOR-org-Complexing-agents-10.pdf (accessed on 20 January 2020).
- International Organization for Standardization. Water Quality-Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry; ISO 11885:2007; IOS: Geneva, Switzerland, 2007. [Google Scholar]
- Hassaan, M.A.; El Nemr, A.; Madkour, F.F. Testing the advanced oxidation processes on the degradation of Direct Blue 86 dye in wastewater. Egypt. J. Aquat. Res. 2016, 43, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Snell, T.W.; Persoone, G. Acute toxicity bioassays using rotifers. I. A test for brackish and marine environment with Brachionus Plicatilis. Aquat. Toxicol. 1989, 14, 65–80. [Google Scholar] [CrossRef]
- Thomas, M.; Zdebik, D. Treatment of Real Textile Wastewater by Using Potassium Ferrate(VI) and Fe(III)/H2O2. Application of Aliivibrio fischeri and Brachionus plicatilis Tests for Toxicity Assessment. Fibres Text. East. Eur. 2019, 3, 78–84. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Treatability Manual Volume III Technologies for Comntrol/Removal of Pollutants, Office of Research and Development; USEPA: Washington, DC, USA, 1981.
- Regulation of the Minister of Construction on the manner of fulfilling the obligations of industrial wastewater suppliers and the conditions of introduction of wastewater to sewage systems. 2016; 1757, 1–9. (In Polish)
- Singh, K.; Bhatia, P.G.; Gupta, R.D. Direct Oxidimetric determination of thiocarbonate sulfur with ferricyanide using iron(II)-dimethylglyoxime or sodium nitroprusside as indicator. Talanta 1982, 29, 47–48. [Google Scholar] [CrossRef]
- Singh, K.; Fodeke, B.A. Determination of thiocarbonate in the presence of sulphite, thiosulphate and thiocyanate by titration with o-hydroxymercuribenzoate. Talanta 1983, 30, 693–694. [Google Scholar] [CrossRef]
- Wroński, M. Determination of sulphide, polysulphide, thiocarbonates and sulphur by extraction with tributyltin hydroxide and of thiosulphate by hydrogenation. Talanta 1981, 28, 173–176. [Google Scholar] [CrossRef]
- Chen, T.-C.; Priambodo, R.; Huang, R.-L.; Huang, Y.-H. The effective electrolytic recovery of dilute copper from industrial wastewater. J. Waste Manag. 2013, 2013, 164780. [Google Scholar] [CrossRef] [Green Version]
- Jon, M.; Heuss-Assbichler, S.; Ullrich, A. Recovery of Zn from wastewater of zinc plating industry by precipitation of doped ZnO nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 2127–2134. [Google Scholar] [CrossRef] [Green Version]
- Tunay, O.; Kabdasli, N.I. Hydroxide precipitation of complexed metals. Water Res. 1994, 28, 2117–2124. [Google Scholar] [CrossRef]
- Beiramzadeh, Z.; Baqersad, M.; Aghababaei, M. Application of the Response Surface Methodology (RSM) in Heavy Metal Removal from Real Power Plant Wastewater Using Electrocoagulation. Available online: https://0-doi-org.brum.beds.ac.uk/10.1080/19648189.2019.1640139 (accessed on 19 December 2020).
- Uzun, Y.; Sahan, T. Optimization with Response Surface Methodology of biosorption conditions of Hg(II) ions from aqueous media by Polyporus squamosus fungi as a new biosorbent. Arch. Environ. Prot. 2017, 43, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Bakar, A.F.A.; Halim, A.A.; Hanafiah, M.M. Optimization of Coagulation-Flocculation Process for Automotive Wastewater Treatment using Response Surface Methodology. Nat. Environ. Pollut. 2015, 14, 567–572. [Google Scholar]
- Summary Report Control and Treatment Technology for the Metal Finishing Industry. Sulfide Precipitation; Industrial Environmental Research Laboratory: Cincinnati, OH, USA, 1980.
- Thomas, M.; Kozik, V.; Barbusiński, K.; Sochanik, A.; Jampilek, J.; Bąk, A. Potassium Ferrate (VI) as the Multifunctional Agent in the Treatment of Landfill Leachate. Molecules 2020, 13, 5017. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Brauch, H.-J. Impact of Aminocarboxylates on Aquatic Organisms and Eutrophication: Overview of Available Data. Environ. Toxicol. 2004, 19, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Siek, M.; Gęca, M.; Hubicki, Z. Role of Chelating Agents of a New Generation in Sorption of Metal Ions. Wastewater Treatment. In Proceedings of the 15th ICHMET, Gdańsk, Poland, 19–23 September 2010. [Google Scholar]
- Bielański, A. Fundamentals of Inorganic Chemistry; PWN: Warsaw, Poland, 2020. [Google Scholar]
- Lipiec, T.; Szmal, Z. Analytical Chemistry with the Basics of Instrumental Analysis; PZWL: Warsaw, Poland, 1996. [Google Scholar]
- Subbiah, R.M.; Sastry, C.A.; Agamuthu, P. Removal of Zink from Rubber Thread Manufacturing Industry Wastewater using Chemical Precipitation/Flocculant. Environ. Prog. 2000, 19, 299–304. [Google Scholar] [CrossRef]
- Jiang, S.; Qu, J.; Xiong, Y. Removal of chelated copper from wastewaters by Fe2+-based replacement-precipitation. Environ. Chem. Lett. 2010, 8, 339–342. [Google Scholar] [CrossRef]
- El-Gohary, F.; Lasheen, M.R.; Abdel-Shafy, H.I. Trace metal removal from wastewater via chemical treatment. In Proceedings of the 1st International Conference Management and Control of Heavy Metals in the Environment, London, UK, September 1979. [Google Scholar]
- Gvozdov, A.O. Phototaxis as a test function in bioassay. Hydrobiol. J. 1986, 22, 65–68. [Google Scholar]





| Run | Experimental Conditions | Experimental Results * | ||
|---|---|---|---|---|
| pH | Na2CS3 (mg) | Time (min) | ∑Cu,Cd,Zn (mg/L) | |
| 1 | 8.0 | 300 | 10 | 2.99 ± 0.30 |
| 2 | 8.0 | 300 | 30 | 2.79 ± 0.28 |
| 3 | 8.0 | 500 | 10 | 1.85 ± 0.19 |
| 4 | 8.0 | 500 | 30 | 1.70 ± 0.17 |
| 5 | 10.0 | 300 | 10 | 0.94 ± 0.09 |
| 6 | 10.0 | 300 | 30 | 0.90 ± 0.09 |
| 7 | 10.0 | 500 | 10 | 0.68 ± 0.07 |
| 8 | 10.0 | 500 | 30 | 0.57 ± 0.06 |
| 9 | 7.3 | 400 | 20 | 3.20 ± 0.32 |
| 10 | 10.7 | 400 | 20 | 0.80 ± 0.08 |
| 11 | 9.0 | 232 | 20 | 0.84 ± 0.08 |
| 12 | 9.0 | 568 | 20 | 0.67 ± 0.07 |
| 13 | 9.0 | 400 | 3 | 0.74 ± 0.07 |
| 14 | 9.0 | 400 | 37 | 0.72 ± 0.07 |
| 15 (C) ** | 9.0 | 400 | 20 | 0.75 ± 0.08 |
| 16 (C) ** | 9.0 | 400 | 20 | 0.76 ± 0.08 |
| Parameter | Unit | Result * |
|---|---|---|
| Color | − | Intense red |
| pH | − | >13 |
| Specific density at 19 ℃ | g/mL | 1.319 ± 0.001 |
| Na2CS3 content | % | 40.8 ± 0.4 |
| Parameter | Unit | Result * |
|---|---|---|
| pH | − | 3.4 ± 0.1 |
| Electrical conductivity, EC | µS/cm | 25,800 ± 1290 |
| Salinity as NaCl | mg/L | 12,850 ± 640 |
| Complexing compounds as EDTA | mg/L | 180 ± 28 |
| Copper (Cu) | mg/L | 59.0 ± 5.90 |
| Cadmium (Cd) | mg/L | 4.50 ± 0.45 |
| Zink (Zn) | mg/L | 22.70 ± 2.27 |
| Parameter | Evaluation of the Effects, ∑Cu,Cd,Zn, mg/L, R2 = 0.9119, R2adj = 0.8532, 3 Parameters, 1 Block, 16 Experiments, MS = 0.1219 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Standard Error | p-Value * | −95% Confidence Interval | +95% Confidence Interval | Factor | Standard Error of Factor | Lower Confidence Interval | Upper Confidence Interval | |
| Constant Value | 0.7197 | 0.2461 | 0.01693 | 0.1628 | 1.2765 | 0.7197 | 0.2461 | 0.1628 | 1.2765 |
| pH (L) | −1.5049 | 0.1889 | 0.00002 | −1.9324 | −1.0775 | −0.7525 | 0.0945 | −0.9662 | −0.5388 |
| pH (Q) | 1.0509 | 0.2294 | 0.00133 | 0.5320 | 1.5699 | 0.5255 | 0.1147 | 0.2660 | 0.7850 |
| Na2CS3 (L) | −0.4548 | 0.1889 | 0.03942 | −0.8823 | −0.0274 | −0.2274 | 0.0945 | −0.4411 | −0.0137 |
| Na2CS3 (Q) | 0.1706 | 0.2294 | 0.47608 | −0.3484 | 0.6896 | 0.0853 | 0.1147 | −0.1742 | 0.3448 |
| Time (L) | −0.0781 | 0.1889 | 0.68885 | −0.5056 | 0.3493 | −0.0391 | 0.0945 | −0.2528 | 0.1746 |
| Time (Q) | 0.1529 | 0.2294 | 0.52178 | −0.3660 | 0.6719 | 0.0765 | 0.1147 | −0.1830 | 0.3359 |
| Parameter | Assessment of Effects, ∑Cu,Cd,Zn, mg/L, R2 = 0.9119, R2adj = 0.8532, 3 Parameters, 1 Block, 16 Experiments, MS = 0.1219 | |||
|---|---|---|---|---|
| SS | MS | F | p-Value * | |
| pH (L) | 7.7326 | 7.7326 | 63.4384 | 0.00002 |
| pH (Q) | 2.5580 | 2.5580 | 20.9860 | 0.00133 |
| Na2CS3 (L) | 0.7064 | 0.7064 | 5.7950 | 0.03942 |
| Na2CS3 (Q) | 0.0674 | 0.0674 | 0.5530 | 0.47608 |
| Time (L) | 0.0209 | 0.0209 | 0.1711 | 0.68885 |
| Time (Q) | 0.0542 | 0.0542 | 0.4443 | 0.52178 |
| Error | 1.0970 | 0.1219 | - | - |
| Predictor | Regression Coefficient | Standard Error | t−Value, df ** = 9 | p–Value *** | −95% Confidence Interval | +95% Confidence Interval |
|---|---|---|---|---|---|---|
| Intercept | 52.71329 | 10.26366 | 5.13592 | 0.00061 | 29.49528 | 75.93130 |
| pH (L) | −10.21092 | 2.06685 | −4.94032 | 0.00080 | −14.88646 | −5.53537 |
| pH (Q) | 0.52547 | 0.11471 | 4.58105 | 0.00133 | 0.26599 | 0.78495 |
| Na2CS3 (L) | −0.00910 | 0.00922 | −0.98623 | 0.34979 | −0.02997 | 0.01177 |
| Na2CS3 (Q) | 0.00001 | 0.00001 | 0.74361 | 0.47608 | −0.00002 | 0.00003 |
| Time (L) | −0.03449 | 0.04684 | −0.73627 | 0.48032 | −0.14046 | 0.07148 |
| Time (Q) | 0.00076 | 0.00115 | 0.66655 | 0.52178 | −0.00183 | 0.00336 |
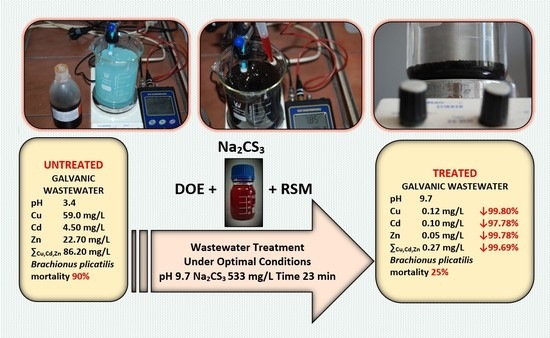
| Parameter * | Before Treatment | After Treatment by Using Na2CS3 (pH 9.7, Na2CS3 533 mg/L, Time 23 min) ** | Removal, % *** | After Treatment by Using NaOH (Alkalization up to pH 11) | Removal, % *** | After Treatment by Using 15% Suspension of Ca(OH)2 (Alkalization up to pH 11) | Removal, % *** | After Treatment by Using 15% Suspension of CaO (Alkalization up to pH 11) | Removal, % *** |
|---|---|---|---|---|---|---|---|---|---|
| pH | 3.4 ± 0.1 | 9.7 ± 0.1 | - | 11.0 ± 0.1 | - | 11.1 ± 0.1 | - | 11.1 ± 0.1 | - |
| Copper (Cu), mg/L | 59.0 ± 5.90 | 0.12 ± 0.01 | 99.80 | 2.50 ± 0.25 | 95.76 | 2.9 ± 0.29 | 95.08 | 2.8 ± 0.28 | 95.25 |
| Cadmium (Cd), mg/L | 4.50 ± 0.45 | 0.10 ± 0.01 | 97.78 | 0.8 ± 0.08 | 82.22 | 0.50 ± 0.05 | 88.89 | 0.42 ± 0.04 | 90.67 |
| Zink (Zn), mg/L | 22.70 ± 2.27 | 0.05 ± 0.01 | 99.78 | 4.6 ± 0.46 | 79.74 | 2.1 ± 0.21 | 90.75 | 2.2 ± 0.22 | 90.31 |
| ∑Cu,Cd,Zn, mg/L | 86.20 ± 8.62 | 0.27 ± 0.03 | 99.69 | 7.90 ± 0.79 | 90.84 | 5.50 ± 0.55 | 93.97 | 5.42 ± 0.54 | 93.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, M.; Kozik, V.; Bąk, A.; Barbusiński, K.; Jazowiecka-Rakus, J.; Jampilek, J. Removal of Heavy Metal Ions from Wastewaters: An Application of Sodium Trithiocarbonate and Wastewater Toxicity Assessment. Materials 2021, 14, 655. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14030655
Thomas M, Kozik V, Bąk A, Barbusiński K, Jazowiecka-Rakus J, Jampilek J. Removal of Heavy Metal Ions from Wastewaters: An Application of Sodium Trithiocarbonate and Wastewater Toxicity Assessment. Materials. 2021; 14(3):655. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14030655
Chicago/Turabian StyleThomas, Maciej, Violetta Kozik, Andrzej Bąk, Krzysztof Barbusiński, Joanna Jazowiecka-Rakus, and Josef Jampilek. 2021. "Removal of Heavy Metal Ions from Wastewaters: An Application of Sodium Trithiocarbonate and Wastewater Toxicity Assessment" Materials 14, no. 3: 655. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14030655