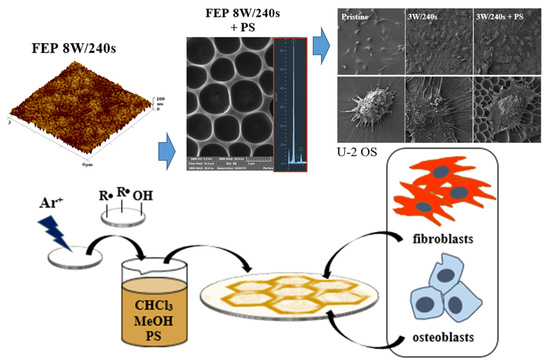
Cell Behavior of Primary Fibroblasts and Osteoblasts on Plasma-Treated Fluorinated Polymer Coated with Honeycomb Polystyrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Substrates
2.3. Characterization of Substrates
2.4. Cell Culture
2.5. Cell Viability
2.6. Cell Seeding for Microscopy Analysis
2.7. Fluorescence Microscopy of MRC-5 and U-2 OS Cells
2.8. SEM of MRC-5 and U-2 OS Cells
3. Results
3.1. Surface Morphology, Roughness, and Surface Area
3.2. The Thickness of Prepared Substrates
3.3. Surface Chemistry
3.4. Surface Wettability and Aging
3.5. Cytocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vacanti, C.A. The history of tissue engineering. J. Cell Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Neděla, O.; Slepička, P.; Švorčík, V. Surface modification of polymer substrates for biomedical applications. Materials 2017, 10, 1115. [Google Scholar] [CrossRef] [PubMed]
- Žáková, P.; Kasálková, N.S.; Slepička, P.; Kolská, Z.; Karpíšková, J.; Stibor, I.; Švorčík, V. Cytocompatibility of polyethylene grafted with triethylenetetramine functionalized carbon nanoparticles. Appl. Surf. Sci. 2017, 422, 809–816. [Google Scholar] [CrossRef]
- Kasálková, N.S.; Slepička, P.; Kolská, Z.; Sajdl, P.; Bačáková, L.; Rimpelová, S.; Švorčík, V. Cell adhesion and proliferation on polyethylene grafted with Au nanoparticles. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam 2012, 272, 391–395. [Google Scholar] [CrossRef]
- Bačáková, L.; Filová, E.; Pařízek, M.; Ruml, T.; Švorčík, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotech. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloid Surf. B-Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Nanotechnology-based biomaterials for orthopaedic applications: Recent advances and future prospects. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110154. [Google Scholar] [CrossRef]
- Qiu, Z.Y.; Cui, Y.; Wang, X.M. Natural bone tissue and its biomimetic. In Mineralized Collagen Bone Graft Substitutes; Wang, X.M., Qiu, Z.Y., Cui, H., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 1–22. [Google Scholar]
- Baptista, D.; Teixeira, L.; van Blitterswijk, C.; Giselbrecht, S.; Truckenmüller, R. Overlooked? Underestimated? Effects of substrate curvature on cell behavior. Trends Biotechnol. 2019, 37, 838–854. [Google Scholar] [CrossRef] [PubMed]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact. Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Review: Mammalian cell behavior on hydrophobic substrates: Influence of surface properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Donoso, M.G.; Méndez-Vilas, A.; Bruque, J.M.; González-Martin, M.L. On the relationship between common amplitude surface roughness parameters and surface area: Implications for the study of cell–material interactions. Int. Biodeter. Biodegr. 2007, 59, 245–251. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarette, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration ant the role of microroughness and nanostructures? Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammons, R.L.; Lumbikanonda, N.; Gross, M.; Cantzler, P. Comparison of osteoblast spreading on microstructured dental implant surfaces and cell behaviour in an explant model of osseointegration. A scanning electron microscopic study. Clin. Oral. Implants Res. 2005, 16, 657–666. [Google Scholar] [CrossRef]
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue. Int. 2002, 71, 519–529. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and engineering the cell surface interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef]
- Chung, T.W.; Liu, D.Z.; Wang, S.Y.; Wang, S.S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Review: Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 6, 66–78. [Google Scholar] [CrossRef]
- Rahmati, M.; Silva, E.A.; Reseland, J.E.; Heyward, C.A.; Haugen, H.J. Biological responses to physicochemical properties of biomaterial surface. Chem. Soc. Rev. 2020, 49, 5178. [Google Scholar] [CrossRef]
- Metwally, S.; Stachewicz, U. Surface potential and charges impact on cell responses on biomaterials interfaces for medical applications. Mater. Sci. Eng. C. 2019, 104, 109883. [Google Scholar] [CrossRef]
- Masui, M.; Takata, H.; Kominami, T. Cell adhesion and the negative cell surface charges in embryonic cells of the starfish Asterina pectinifera. Electrophoresis 2002, 23, 2087–2095. [Google Scholar] [CrossRef]
- Stoltz, J.F.; Boisseau, M.; Muller, S.; Wang, X.; Legrand, S.; Labrador, M.V. Hemorheology and vascular endothelial cells. J. Mal. Vasc. 1999, 24, 99–109. [Google Scholar] [PubMed]
- Lee, J.H.; Jung, H.W.; Kang, I.K.; Lee, H.B. Cell behaviour on polymer surfaces with different functional groups. Biomaterials 1994, 15, 705–711. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Liu, L.; Chen, S.; Giachelli, C.M.; Ratner, B.D.; Jiang, S. Controlling osteopontin orientation on surfaces to modulate endothelial cell adhesion. J. Biomed. Mater. Res. A 2005, 74, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jones, M.D.; Davidson, M.G.; Chaudhuri, J.B.; Ellis, M.J. Surfactant-free poly (lactide-co-glycolide) honeycomb films for tissue engineering: Relating solvent, monomer ratio and humidity to scaffold structure. Biotechnol. Lett. 2011, 33, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabu, H. Fabrication of honeycomb films by the breath figure technique and their applications. Sci. Technol. Adv. Mater. 2018, 19, 802–822. [Google Scholar] [CrossRef] [Green Version]
- Sunami, H.; Ito, E.; Tanaka, M.; Yamamoto, S.; Shimomura, M. Effect of honeycomb film on protein adsorption, cell adhesion and proliferation. Colloid Surf. A-Physicochem. Eng. Asp. 2006, 284, 548–551. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, T.; Ookura, R.; Nishida, J.; Arai, K.; Hayashi, J.; Kurono, N.; Shimomura, M. Fabrication of honeycomb film of an amphiphilic copolymer at the air−water interface. Langmuir 2002, 18, 5734–5740. [Google Scholar] [CrossRef]
- Gentsch, R.; Börner, H.G. Designing three-dimensional materials at the interface to biology. In Bioactive Surfaces; Börner, H.G., Lutz, J.F., Eds.; Springer: Heidelberg, Germany, 2010; pp. 163–192. [Google Scholar]
- Dong, C.; Hao, J. Honeycomb films with ordered patterns and structures. In Comprehensive Supramolecular Chemistry II; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S. Regulating MC3T3-E1 cells on deformable poly (ε-caprolactone) honeycomb films prepared using a surfactant-free breath figure method in a water-miscible solvent. ACS Appl. Mater. Interfaces 2012, 4, 4966–4975. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T.; Sato, M.; Yabu, H.; Shimomura, M. Honeycomb-shaped surface topography induces differentiation of human mesenchymal stem cells (hMSCs): Uniform porous polymer scaffolds prepared by the breath figure technique. Biomater. Sci. 2014, 2, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Widawski, G.; Rawiso, M.; François, B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- Yang, S.Y.; Kim, E.S.; Jeon, G.; Choi, K.Y.; Kim, J.K. Enhanced adhesion of osteoblastic cells on polystyrene films by independent control of surface topography and wettability. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013, 33, 1689–1695. [Google Scholar] [CrossRef]
- Guo, L.; Kawazoe, N.; Hoshiba, T.; Tateishi, T.; Chen, G.; Zhang, X. Osteogenic differentiation of human mesenchymal stem cells on chargeable polymer-modified surfaces. J. Biomed. Mater. Res. A 2008, 87, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.; Frischauf, I.; Olbrich, M.; Peterbauer, T.; Hering, S.; Preiner, J.; Hinterdorfer, P.; Romanin, C.; Heitz, J. Proliferation of aligned mammalian cells on laser-nanostructured polystyrene. Biomaterials 2008, 29, 1796–1806. [Google Scholar] [CrossRef]
- Sun, L.; Pereira, D.; Wang, Q.; Barata, D.B.; Truckenmüller, R.; Li, Z.; Xu, X.; Habibovic, P. Controlling growth and osteogenic differentiation of osteoblasts on microgrooved polystyrene surfaces. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The evolution of polystyrene as a cell culture material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.; Shen, X.; Liu, X.; Chen, Z.; Shu, B.; Wan, L.; He, J. Hybrid breath figure method: A new insight in Petri dishes for cell culture. J. Colloid Interface Sci. 2019, 541, 114–122. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Naviglio, S.; Barrino, F. Antibacterial properties of sol–gel biomaterials with different percentages of PEG or PCL. Macromol. Symp. 2020, 389, 1900056. [Google Scholar] [CrossRef]
- Yoshida, S.; Hagiwara, K.; Hasebe, T.; Hotta, A. Surface modification of polymers by plasma treatments for the enhancement of biocompatibility and controlled drug release. Surf. Coat. Technol. 2013, 233, 99–107. [Google Scholar] [CrossRef]
- Gangal, S.V.; Brothers, P.D. Perfluorinated polymers, perfluorinated ethylene–propylene copolymers. In Encyclopedia of Polymer Science and Technology, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 1–15. [Google Scholar]
- Slepička, P.; Peterková, L.; Rimpelová, S.; Pinkner, A.; Kasálková, N.S.; Kolská, Z.; Švorčík, V. Plasma activated perfluoroethylenepropylene for cytocompatibility enhancement. Polym. Degrad. Stab. 2016, 130, 277–287. [Google Scholar] [CrossRef]
- Peterková, L.; Rimpelová, S.; Křížová, I.; Slepička, P.; Kasálková, N.S.; Švorčík, V.; Ruml, T. Biocompatibility of Ar plasma-treated fluorinated ethylene propylene: Adhesion and viability of human keratinocytes. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 100, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Peterková, L.; Rimpelová, S.; Slepička, P.; Křížová, I.; Kasálková, N.S.; Švorčík, V.; Ruml, T. Argon plasma-treated fluorinated ethylene propylene: Growth of primary dermal fibroblasts and mesenchymal stem cells. Tissue Cell 2019, 58, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Neznalová, K.; Fajstavr, D.; Slepičková Kasálková, N.; Švorčík, V. Honeycomb-like pattern formation on perfluoroethylenepropylene enhanced by plasma treatment. Plasma Process. Polym. 2019, 16, 1900063. [Google Scholar] [CrossRef]
- Slepička, P.; Trostová, S.; Slepičková Kasálková, N.; Kolská, Z.; Sajdl, P.; Švorčík, V. Surface modification of biopolymers by argon plasma and thermal treatment. Plasma Process. Polymers 2012, 9, 197–206. [Google Scholar] [CrossRef]
- Novotná, Z.; Řezníčková, A.; Rimpelová, S.; Veselý, M.; Kolská, Z.; Švorčík, V. Tailoring of PEEK bioactivity for improved cell interaction: Plasma treatment in action. RSC Advances 2015, 5, 41428–41436. [Google Scholar] [CrossRef] [Green Version]
- Juřík, P.; Slepička, P.; Mistrík, J.; Janíček, P.; Rimpelová, S.; Kolská, Z.; Švorčík, V. Oriented gold ripple-like structures on poly-l-lactic acid. Appl. Surf. Sci. 2014, 321, 503–510. [Google Scholar] [CrossRef]
- Bui, V.T.; Tran, Q.C.; Nguyen, V.T.; Dao, V.D.; Choi, J.S.; Choi, H.S. Ordered honeycomb biocompatible polymer films via a one-step solution-immersion phase separation used as a scaffold for cell cultures. Chem. Eng. J. 2017, 320, 561–569. [Google Scholar] [CrossRef]
- Fajstavrová, K.; Rimpelová, S.; Fajstavr, D.; Švorčík, V.; Slepička, P. PLLA Honeycomb-like pattern on fluorinated ethylene propylene as a substrate for fibroblast growth. Polymers 2020, 12, 2436. [Google Scholar] [CrossRef]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajstavrová, K.; Rimpelová, S.; Fajstavr, D.; Švorčík, V.; Slepička, P. Cell Behavior of Primary Fibroblasts and Osteoblasts on Plasma-Treated Fluorinated Polymer Coated with Honeycomb Polystyrene. Materials 2021, 14, 889. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14040889
Fajstavrová K, Rimpelová S, Fajstavr D, Švorčík V, Slepička P. Cell Behavior of Primary Fibroblasts and Osteoblasts on Plasma-Treated Fluorinated Polymer Coated with Honeycomb Polystyrene. Materials. 2021; 14(4):889. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14040889
Chicago/Turabian StyleFajstavrová, Klára, Silvie Rimpelová, Dominik Fajstavr, Václav Švorčík, and Petr Slepička. 2021. "Cell Behavior of Primary Fibroblasts and Osteoblasts on Plasma-Treated Fluorinated Polymer Coated with Honeycomb Polystyrene" Materials 14, no. 4: 889. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14040889