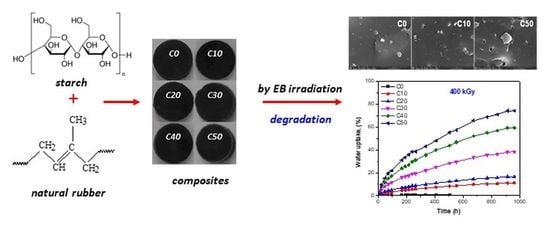
Water Absorption Kinetics in Composites Degraded by the Radiation Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Laboratory Tests
2.2.1. Water Absorption Tests and Measurements
2.2.2. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Water Uptake
3.2. Mechanism of Water Transport
3.3. Transport Coefficients
3.4. Morphological Investigation by Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aatmeeyata; Sharma, M. Polycyclic aromatic hydrocarbons, elemental and organic carbon emissions from tire-wear. Sci. Total Environ. 2010, 408, 4563–4568. [Google Scholar] [CrossRef]
- Adhikari, B.; De, D.; Maiti, S. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Artíñano, B.; Gómez-Moreno, F.J.; Díaz, E.; Amato, F.; Pandolfi, M.; Alonso-Blanco, E.; Coz, E.; García-Alonso, S.; Becerril-Valle, M.; Querol, X.; et al. Outdoor and indoor particle characterization from a large and uncontrolled combustion of a tire landfill. Sci. Total Environ. 2017, 593–594, 543–551. [Google Scholar] [CrossRef]
- Chapiro, A. General consideration of the radiation chemistry of polymers. Nucl. Instrum. Meth. B 1995, 105, 5–7. [Google Scholar] [CrossRef]
- Kornacka, E.M. Radiation-induced oxidation of polymers. In Applications of Ionizing Radiation in Materials Processing, 1st ed.; Yongxia, S., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; Volume 1, pp. 183–192. Available online: http://www.ichtj.waw.pl/ichtj/publ/monogr/sun2017/sun-chapter8.pdf (accessed on 28 January 2021).
- Manaila, E.; Stelescu, M.D.; Craciun, G. Degradation Studies Realized on Natural Rubber and Plasticized Potato Starch Based Eco-Composites Obtained by Peroxide Cross-Linking. Int. J. Mol. Sci. 2018, 19, 2862. [Google Scholar] [CrossRef] [Green Version]
- Rohana Yahya, Y.S.; Azura, A.R.; Ahmad, Z. Effect of Curing Systems on Thermal Degradation Behaviour of Natural Rubber (SMR CV 60). J. Phys. Sci. 2011, 22, 1–14. Available online: http://web.usm.my/jps/22-2-11/22.2.1.pdf (accessed on 26 June 2020).
- Azura, A.R.; Muhr, A.H.; Thomas, A.G. Diffusion and reactions of oxygen during ageing for conventionally cured natural rubber vulcanisate. Polym. Plast. Technol. Eng. 2006, 45, 893–896. [Google Scholar] [CrossRef]
- Valodkar, M.; Thakore, S. Thermal and Mechanical Properties of Natural Rubber and Starch Nanobiocomposites. Int. J. Polym. Anal. Charact. 2010, 15, 387–395. [Google Scholar] [CrossRef]
- Pimolsiriphol, V.; Saeoui, P.; Sirisinha, C. Relationship Among Thermal Ageing Degradation, Dynamic Properties, Cure Systems and Antioxidants in Natural Rubber Vulcanisates. Polym. Plast. Technol. Eng. 2007, 46, 113–121. [Google Scholar] [CrossRef]
- Spadaro, G.; Alessi, S.; Dispenza, C. Ionizing radiation-induced crosslinking and degradation of polymers. In Applications of Ionizing Radiation in Materials Processing, 1st ed.; Yongxia, S., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warsaw, Poland, 2017; Volume 1, pp. 167–182. Available online: http://www.ichtj.waw.pl/ichtj/publ/monogr/sun2017/sun-chapter7.pdf (accessed on 29 January 2021).
- Gani, A.; Gazanfar, T.; Jan, R.; Wani, S.M.; Masoodi, F.A. Effect of gamma irradiation on the physicochemical and morphological properties of starch extracted from lotus stem harvested from Dal lake of Jammu and Kashmir, India. J. Saudi Soc. Agric. Sci. 2013, 12, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Nemtanu, M.R.; Brasoveanu, M. Radio-sensitivity of some starches treated with, accelerated electron beam. Starch Starke 2012, 64, 435–440. [Google Scholar] [CrossRef]
- Brasoveanu, M.; Nemtanu, M.R. Aspects on starches modified by ionizing radiation processing. In Applications of Modified Starches, 1st ed.; Flores-Huicochea, E., Rendon-Villalobos, R., Eds.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/books/applications-of-modified-starches/aspects-on-starches-modified-by-ionizing-radiation-processing (accessed on 2 February 2021).
- Kamal, H.; Sabry, G.M.; Lotfy, S.; Abdallah, N.M.; Ulanski, P.; Rosiak, J.; Hegazy, S.A. Controlling of Degradation Effects in Radiation Processing of Starch. J. Macromol. Sci. A 2007, 44, 865–875. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Ighigeanu, D.; Stelescu, M.D. A method to improve the characteristics of EPDM rubber based eco-composites with electron beam. Polymers 2020, 12, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manaila, E.; Craciun, G.; Ighigeanu, D.; Lungu, I.B.; Dumitru, M.; Stelescu, M.D. Electron Beam Irradiation: A method for degradation of composites based on natural rubber and plasticized starch. Polymers 2021, 13, 1950. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G.; Ighigeanu, D. Wood Sawdust/Natural Rubber Ecocomposites Cross-Linked by Electron Beam Irradiation. Materials 2016, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Craciun, G.; Manaila, E.; Stelescu, M.D. New Elastomeric Materials Based on Natural Rubber Obtained by Electron Beam Irradiation for Food and Pharmaceutical Use. Materials 2016, 9, 999. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, H.N.; Zhang, Z.Y.; Richardson, M.O.W. Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos. Sci. Technol. 2007, 67, 1674–1683. [Google Scholar] [CrossRef]
- Moustafa, A.B.; Mounir, R.; El Miligy, A.A.; Mohamed, M.A. Effect of gamma irradiation on the properties of natural rubber/styrene butadiene rubber blends. Arab. J. Chem. 2016, 9, S124–S129. [Google Scholar] [CrossRef] [Green Version]
- Kruželak, J.; Sykora, R.; Hudec, I. Peroxide vulcanization of natural rubber. Part I: Effect of temperature and peroxide concentration. J. Polym. Eng. 2014, 34, 617–624. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Sasithornsonti, Y.; Phinyocheep, P. Green natural rubber-g-modified starch for controlling urea release. Carbohyd. Polym. 2012, 89, 251–258. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef]
- Takeda, C.; Takeda, Y.; Hizukuri, S. Structure of amylomaize amylase. Cereal Chem. 1989, 66, 22–25. Available online: https://www.cerealsgrains.org/publications/cc/backissues/1989/Documents/66_22.pdf (accessed on 2 February 2021).
- Singh, N.; Nakaura, Y.; Inouchi, N.; Nishinari, K. Fine structure, thermal and viscoelastic properties of starches separated from indica rice cultivars. Starch Starke 2007, 59, 10–20. [Google Scholar] [CrossRef]
- Singh, N.; Isono, N.; Srichuwong, S.; Noda, T.; Nishinari, K. Structural, thermal and viscoelastic properties of potato starches. Food Hydrocoll. 2008, 22, 979–988. [Google Scholar] [CrossRef]
- Singh, N.; Nakaura, Y.; Inouchi, N.; Nishinari, K. Structure and viscoelastic properties of starches separated from different legumes. Starch Starke 2008, 60, 349–357. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, thermal and functional properties of gamma irradiated chickpea starch. Int. J. Biol. Macromol. 2017, 97, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Stelescu, M.D.; Manaila, E.; Craciun, G.; Georgescu, M. Aspects Regarding Ageing of Compounds Based on Natural Rubber and Plasticized Starch. Mater. Plast. 2018, 55, 351–356. Available online: https://revmaterialeplastice.ro/pdf/21%20STELESCU%203%2018.pdf (accessed on 5 February 2021). [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of Plasticizer Type and Concentration on Tensile, Thermal and Barrier Properties of Biodegradable Films Based on Sugar Palm (Arenga pinnata) Starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Morales, N.J.; Pérez, E.; Tapia, M.S.; Famá, L. Physico-chemical properties of edible films derived from native and phosphated cush-cush yam and cassava starches. Food Packag. Shelf Life 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R.; León, A.E. Properties of triticale protein films and their relation to plasticizing–antiplasticizing effects of glycerol and sorbitol. Ind. Crop. Prod. 2013, 50, 297–303. [Google Scholar] [CrossRef]
- Pimpa, B.; Muhammad, S.K.S.; Hassan, M.A.; Ghazali, Z.; Hashim, K.; Kanjanasopa, D. Effect of electron beam irradiation on physicochemical properties of sago starch. Songklanakarin J. Sci. Technol. 2007, 29, 759–768. Available online: http://doc2.clib.psu.ac.th/public14/article2/research/290326.pdf (accessed on 7 February 2021).
- Rosenthal, I. Electromagnetic Radiations in Food Science; Advanced Series in Agricultural Sciences, 19; Softcover Reprint of the Original 1st ed.; Springer: New York, NY, USA, 1992; pp. 1–19. [Google Scholar]
- Radley, J.A. The effect of irradiation by high energy cathod rays on starch. Starch Starke 1960, 12, 201–203. [Google Scholar] [CrossRef]
- El Saadany, M.A.; El Fatah, A.; El Safti, A.; El Saadany, M. Effect of gamma irradiation on Egyption sweet potato starch. Starch Starke 1974, 26, 190–195. [Google Scholar] [CrossRef]
- Rayas Duartz, P.; Rupnow, J.H. Gamma-irradiated dry bean (Phaseolus vulgaris) starch: Physicochemical properties. J. Food Sci. 1994, 59, 839–843. [Google Scholar] [CrossRef]
- Kumar, K.A.A.; Sreekala, M.S.; Arun, S. Studies on Properties of Bio-Composites from Ecoflex/Ramie Fabric-Mechanical and Barrier Properties. J. Biomater. Nanobiotechnol. 2012, 3, 396–404. Available online: https://www.scirp.org/pdf/JBNB20120300010_39760283.pdf (accessed on 22 March 2021). [CrossRef] [Green Version]
- Jacob, M.; Varughese, K.T.; Thomas, S. Water Sorption Studies of Hybrid Biofiber-Reinforced Natural Rubber Biocomposites. Biomacromolecules 2005, 6, 2969–2979. [Google Scholar] [CrossRef]
- Mathew, L.; Joseph, K.U.; Joseph, R. Swelling behaviour of isora/natural rubber composites in oils used in Automobiles. Bull. Mater. Sci. 2006, 29, 91–99. Available online: https://0-link-springer-com.brum.beds.ac.uk/content/pdf/10.1007/BF02709362.pdf (accessed on 22 March 2021). [CrossRef]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. Part. A Appl. Sci. Manuf. 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Ehi, I.P.; Bidemi, J.K.; Yahaya, L.E. Kinetic Studies on Water Absorption properties of Cocoa-pod Epoxy Composites. Iran. Iran. J. Energy Environ. 2016, 7, 48–51. Available online: https://pdfcookie.com/documents/kinetic-studies-on-water-absorption-properties-of-cocoa-pod-epoxy-composites-w5lqe4gr6ql7 (accessed on 28 January 2020).
- Barrer, R.M.; Barrie, J.A.; Rogers, M.G. Heterogeneous membranes: Diffusion in filled rubber. J. Polym. Sci. Part A 1963, 1, 2565–2586. [Google Scholar] [CrossRef]
- Maria, H.J.; Lyczko, N.; Nzihou, A.; Mathew, C.; George, S.C.; Joseph, K.; Thomas, S. Transport of organic solvents through natural rubber/nitrile rubber/organically modified montmorillonite nanocomposites. J. Mater. Sci. 2013, 48, 5373–5386. Available online: https://0-link-springer-com.brum.beds.ac.uk/content/pdf/10.1007/s10853-013-7332-7.pdf (accessed on 28 January 2020). [CrossRef] [Green Version]
- Kumar, S.A.; Kumaran, M.G.; Thomas, S. Sorption and diffusion of arenes through poly (ethylene-co-vinyl acetate) membranes. J. Mater. Sci. 2006, 41, 4892–4900. Available online: https://0-link-springer-com.brum.beds.ac.uk/content/pdf/10.1007/s10853-006-0315-1.pdf (accessed on 25 January 2021). [CrossRef]
- Aminabhavi, T.M.; Phayde, H.T.S. Molecular-transport characteristics of santoprene thermoplastic rubber in the presence of aliphatic alkanes over the temperature interval of 25 °C to 70 °C. Polymer 1995, 36, 1023–1033. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Khinnavar, R.S. Diffusion and sorption of organic liquids through polymer membranes: 10. polyurethane, nitrile-butadiene rubber and epichlorohydrin versus aliphatic-alcohols (C1–C5). Polymer 1993, 34, 1006–1008. [Google Scholar] [CrossRef]
- Anil Kumar, P.V.; Varughese, K.T.; Thomas, S. Molecular Transport of Aromatic Hydrocarbons through Ethylene Propylene Diene Terpolymer. Polym. Polym. Compos. 2002, 10, 553–565. [Google Scholar] [CrossRef]
- Harogoppad, S.B.; Aminabhavi, T.M. Diffusion and sorption of organic liquids through polymer membranes. 5. Neoprene, styrene-butadiene-rubber, ethylene-propylene-diene terpolymer, and natural rubber versus hydrocarbons (C8–C16). Macromolecules 1991, 24, 2598–2605. [Google Scholar] [CrossRef]
- Mathew, A.P.; Pakirisamy, S.; Kumaran, M.G.; Thomas, S. Transport of styrene monomer through natural rubber. Polymer 1995, 36, 4935–4942. [Google Scholar] [CrossRef]
- Kumar, S.A.; Thomas, S.; Kumaran, M.G. Transport of aromatic hydrocarbons through poly(ethylene-co-vinyl acetate) membranes. Polymer 1997, 38, 4629–4640. [Google Scholar] [CrossRef]
- Jacob, A.; Kurian, P.; Aprem, A.S. Transport Properties of Natural Rubber Latex Layered Clay Nanocomposites. J. Appl. Polym. Sci. 2008, 108, 2623–2629. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.R. Pasting properties modeling and comparative analysis of starch exposed to ionizing radiation. Radiat. Phys. Chem. 2002, 168, 108492. [Google Scholar] [CrossRef]
- Lomelí Ramírez, M.G.; Satyanarayana, K.G.; Iwakiri, S.; Bolzon de Muniz, G.; Tanobe, V.; Sydenstricker Flores-Sahagun, T. Study of the properties of biocomposites. Part I. Cassava starch-green coir fibers from Brazil. Carbohydr. Polym. 2011, 86, 1712–1722. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.F.; Peng, Z.; Li, S.D.; Lin, H.; Zhang, K.X.; She, X.D.; Fu, X. The impact of esterification on the properties of starch/natural rubber composite. Compos. Sci. Technol. 2009, 69, 1797–1803. [Google Scholar] [CrossRef]
- Carvalho, A.J.F.; Job, A.E.; Alves, N.; Curvelo, A.A.S.; Gandini, A. Thermoplastic starch/natural rubber blends. Carbohydr. Polym. 2003, 53, 95–99. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, D.S.; Won, J.S.; Jin, D.Y.; Lee, H.J.; Lee, S.G. Effects of Thermal and Humidity Aging on the Interfacial Adhesion of Polyketone Fiber Reinforced Natural Rubber Composites. Adv. Mater. Sci. Eng. 2016, 2016, 4159072. [Google Scholar] [CrossRef] [Green Version]
- Muniandy, K.; Ismail, H.; Othman, N. Biodegradation, morphological, and FTIR study of rattan powder-filled natural rubber composites as a afunction of filler loading and a silane coupling agent. BioResources 2012, 7, 957–971. Available online: https://bioresources.cnr.ncsu.edu/resources/biodegradation-morphological-and-ftir-study-of-rattan-powder-filled-natural-rubber-composites-as-a-function-of-filler-loading-and-silane-coupling-agent/ (accessed on 11 February 2021).






| Sample Codes | Filler Content (phr) | Equilibrium Sorption (%) | |||
|---|---|---|---|---|---|
| Non Irradiated | 150 kGy | 300 kGy | 450 kGy | ||
| C0 | 0 | 0.69 ± 0.03 | 0.64 ± 0.21 | 0.71 ± 0.02 | 0.74 ± 0.05 |
| C10 | 10 | 11.52 ± 0.47 | 11.20 ± 0.71 | 10.51 ± 0.38 | 11.03 ± 0.53 |
| C20 | 20 | 15.92 ± 0.52 | 17.88 ± 0.22 | 17.31 ± 0.46 | 16.64 ± 1.07 |
| C30 | 30 | 36.24 ± 0.86 | 41.34 ± 1.30 | 38.22 ± 1.88 | 38.17 ± 1.46 |
| C40 | 40 | 60.89 ± 2.54 | 59.44 ± 5.02 | 61.93 ± 1.11 | 59.32 ± 2.94 |
| C50 | 50 | 79.60 ± 3.35 | 87.01 ± 2.87 | 79.19 ± 3.69 | 74.27 ± 3.64 |
| Sample Codes | Filler Content (phr) | Mass Loss (%) | |||
|---|---|---|---|---|---|
| Non Irradiated | 150 kGy | 300 kGy | 450 kGy | ||
| C0 | 0 | 0.274 ± 0.03 | 0.760 ± 0.21 | 0.753 ± 0.23 | 0.7440 ± 74 |
| C10 | 10 | 0.315 ± 0.02 | 1.887 ± 0.04 | 2.058 ± 0.04 | 2.064 ± 0.21 |
| C20 | 20 | 0.333 ± 0.01 | 2.347 ± 0.07 | 2.435 ± 0.04 | 2.480 ± 0.03 |
| C30 | 30 | 1.115 ± 0.02 | 3.335 ± 0.11 | 3.626 ± 0.04 | 3.791 ± 0.04 |
| C40 | 40 | 2.147 ± 0.05 | 3.339 ± 0.03 | 3.447 ± 0.11 | 3.370 ± 0.23 |
| C50 | 50 | 3.087 ± 0.03 | 3.335 ± 0.23 | 3.355 ± 0.45 | 4.009 ± 0.43 |
| Sample Codes | Filler Content (phr) | n | |||
|---|---|---|---|---|---|
| Non Irradiated | 150 kGy | 300 kGy | 450 kGy | ||
| C0 | 0 | 0.733 | 0.727 | 0.746 | 0.741 |
| C10 | 10 | 0.554 | 0.557 | 0.573 | 0.571 |
| C20 | 20 | 0.568 | 0.573 | 0.585 | 0.590 |
| C30 | 30 | 0.579 | 0.575 | 0.583 | 0.590 |
| C40 | 40 | 0.589 | 0.851 | 0.572 | 0.587 |
| C50 | 50 | 0.592 | 0.566 | 0.590 | 0.597 |
| Sample Codes | Filler Content (phr) | k (×10−2) | |||
|---|---|---|---|---|---|
| Non Irradiated | 150 kGy | 300 kGy | 450 kGy | ||
| C0 | 0 | 1.84 | 1.73 | 1.29 | 1.26 |
| C10 | 10 | 4.37 | 4.28 | 3.75 | 3.74 |
| C20 | 20 | 4.16 | 3.85 | 3.61 | 3.57 |
| C30 | 30 | 3.93 | 3.75 | 3.58 | 3.53 |
| C40 | 40 | 3.52 | 3.74 | 3.56 | 3.45 |
| C50 | 50 | 3.42 | 3.72 | 3.43 | 3.39 |
| Sample Codes | Filler Content (phr) | D* (×10−9 m2s−1) | |||
|---|---|---|---|---|---|
| Non Irradiated | 150 kGy | 300 kGy | 450 kGy | ||
| C0 | 0 | 3.257 | 2.729 | 3.195 | 3.346 |
| C10 | 10 | 3.901 | 3.995 | 3.470 | 3.463 |
| C20 | 20 | 4.244 | 4.387 | 4.313 | 4.314 |
| C30 | 30 | 6.335 | 6.313 | 5.942 | 5.947 |
| C40 | 40 | 9.145 | 8.870 | 7.521 | 7.436 |
| C50 | 50 | 10.914 | 9.824 | 11.010 | 11.119 |
| Sample Codes | Filler Content (phr) | S (%) | |||
|---|---|---|---|---|---|
| Non Irradiated | 150 kGy | 300 kGy | 450 kGy | ||
| C0 | 0 | 0.00672 | 0.00762 | 0.00721 | 0.00745 |
| C10 | 10 | 0.112 | 0.115 | 0.109 | 0.110 |
| C20 | 20 | 0.159 | 0.185 | 0.175 | 0.172 |
| C30 | 30 | 0.362 | 0.413 | 0.393 | 0.382 |
| C40 | 40 | 0.581 | 0.612 | 0.625 | 0.626 |
| C50 | 50 | 0.796 | 0.916 | 0.808 | 0.743 |
| Sample Codes | Filler Content (phr) | P (×10−10 m2 s−1) | |||
|---|---|---|---|---|---|
| Non Irradiated | 150 kGy | 300 kGy | 450 kGy | ||
| C0 | 0 | 0.235 | 0.207 | 0.235 | 0.240 |
| C10 | 10 | 4.486 | 4.564 | 3.697 | 3.724 |
| C20 | 20 | 6.880 | 7.578 | 7.395 | 6.952 |
| C30 | 30 | 23.38 | 25.48 | 22.31 | 22.67 |
| C40 | 40 | 53.08 | 49.87 | 46.61 | 47.08 |
| C50 | 50 | 88.68 | 90.01 | 85.02 | 79.21 |
| Sample Codes | Filler (phr) | Non Irradiated | KS (mol kg−1) | ||
|---|---|---|---|---|---|
| 150 kGy | 300 kGy | 450 kGy | |||
| C0 | 0 | 0.362 | 0.341 | 0.376 | 0.392 |
| C10 | 10 | 5.970 | 5.906 | 5.530 | 5.738 |
| C20 | 20 | 8.377 | 9.398 | 9.115 | 8.747 |
| C30 | 30 | 19.08 | 21.76 | 20.11 | 20.08 |
| C40 | 40 | 30.35 | 32.18 | 33.22 | 32.24 |
| C50 | 50 | 41.67 | 46.97 | 41.66 | 39.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaila, E.; Craciun, G.; Ighigeanu, D.; Stelescu, M.D. Water Absorption Kinetics in Composites Degraded by the Radiation Technique. Materials 2021, 14, 4659. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14164659
Manaila E, Craciun G, Ighigeanu D, Stelescu MD. Water Absorption Kinetics in Composites Degraded by the Radiation Technique. Materials. 2021; 14(16):4659. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14164659
Chicago/Turabian StyleManaila, Elena, Gabriela Craciun, Daniel Ighigeanu, and Maria Daniela Stelescu. 2021. "Water Absorption Kinetics in Composites Degraded by the Radiation Technique" Materials 14, no. 16: 4659. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14164659