Densification of a NASICON-Type LATP Electrolyte Sheet by a Cold-Sintering Process
Abstract
:1. Introduction
- Dissolution of ceramics particles from surface into aqueous solution
- Rearrangement of particles
- Formation of a supersaturated solution through the evaporation of the aqueous solution
- Crystal growth and recrystallization of the metastable phase
2. Materials and Methods
3. Results and Discussion
3.1. Wetting Behavior of the LATP Sheet
3.2. Phase Identification
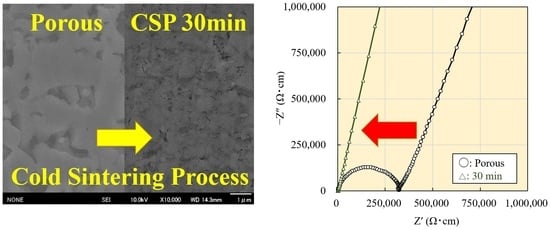
3.3. Cross-Sectional Morphology
3.4. Electrical Conductivity Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsai, C.L.; Roddatis, V.; Chandran, C.V.; Ma, Q.; Uhlendruck, S.; Bram, M.; Heitjans, P.; Guillon, O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, A.; Meyer, H.M.; Nanda, J.; Wolfenstine, J.; Sakamoto, J. Characterizing the Li–Li7La3Zr2O12 interface stability and kinetics as a function of temperature and current density. J. Power Sources 2016, 302, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Porz, L.; Swamy, T.; Sheldon, B.W.; Rettenwander, D.; Fromling, T.; Thaman, H.L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y.M. Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolytes. Adv. Energy Mater. 2017, 7, 1701003. [Google Scholar] [CrossRef]
- Bucharsky, E.C.; Schwll, K.G.; Hingtennach, A.; Hoffmann, M.J. Preparation and characterization of sol–gel derived high lithium ion conductive NZP-type ceramics Li1+xAlxTi2-x(PO4)3. Solid State Ionics 2015, 274, 77–82. [Google Scholar] [CrossRef]
- Chandran, C.V.; Pristat, S.; Witt, E.; Tietz, F.; Heitjans, P. Solid-State NMR Investigations on the Structure and Dynamics of the Ionic Conductor Li1+xAlxTi2–x(PO4)3 (0.0 ≤ x ≤ 1.0). J. Phys. Chem. C 2016, 120, 8436–8442. [Google Scholar] [CrossRef]
- Hupfer, T.; Bucharsky, E.C.; Schell, K.G.; Senyshyn, A.; Monchak, M.; Hoffmann, M.J.; Ehrenberg, H. Evolution of micro-structure and its relation to ionic conductivity in Li1+xAlxTi2-x(PO4)3. Solid State Ionics 2016, 288, 235–239. [Google Scholar] [CrossRef]
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G. Ionic Conductivity of the Lithium Titanium Phosphate (Li1+XMXTi2−X(PO4)3, M = Al, Sc, Y, and La) Systems. J. Electrochem. Soc. 1989, 136, 590–591. [Google Scholar] [CrossRef]
- Cretin, M.; Fabry, P.; Abello, L. Study of Li1+xAlxTi2−x(PO4)3 for Li+ potentiometric sensors. J. Eur. Ceram. Soc. 1995, 15, 1149–1156. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Kumar, B. Water Durable Lithium Ion Conducting Composite Membranes from the Li2O-Al2O3-TiO2-P2O5 Glass-Ceramic. J. Electrochem. Soc. 2007, 154, A331–A336. [Google Scholar] [CrossRef]
- Hasegawa, S.; Imanishi, N.; Zhang, T.; Zie, J.; Hirano, A.; Takeda, Y.; Yamamoto, O. Study on lithium/air secondary batteries—Stability of NASICON-type lithium ion conducting glass-ceramics with water. J. Power Sources 2009, 189, 371–377. [Google Scholar] [CrossRef]
- Fu, J. Superionic conductivity of glass-ceramics in the system Li2O-Al2O3-TiO2-P2O5. Solid State Ionics 1997, 96, 195–200. [Google Scholar] [CrossRef]
- Maria, J.P.; Kang, X.; Floyd, R.D.; Dickey, E.C.; Guo, H.; Guo, J.; Baker, A.; Funihashi, S.; Randall, C.A. Cold sintering: Cur-rent status and prospects. J. Mater. Res. 2017, 32, 3205–3218. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhao, X.; Beauvoir, T.H.D.; Seo, J.H.; Berbano, S.S.; Baker, A.L.; Azina, C.; Randall, C.A. Recent Progress in Applica-tions of the Cold Sintering Process for Ceramic-Polymer Composites. Adv. Funct. Mater. 2018, 28, 1801724. [Google Scholar] [CrossRef]
- Guo, J.; Floyd, R.; Lowum, S.; Maria, J.P.; Beauvoir, T.H.D.; Seo, J.H.; Randall, C.A. Cold Sintering: Progress, Challenges, and Future Opportunities. Annu. Rev. Mater. Res. 2019, 49, 275–295. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Sun, Q.; Wang, D.; Adair, K.R.; Liang, J.; Zhang, C.; Zhang, L.; Lu, S.; Huang, H.; et al. Insight into the Microstructure and Ionic Conductivity of Cold Sintered NASICON Solid Electrolyte for Solid-State Batteries. ACS Appl. Mater. Interfaces 2019, 11, 27890–27896. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lyon, C.K.; Seo, J.H.; Raymond, L.H.; Leng, Y.; Wang, C.Y.; Hickner, M.H.; Randall, C.A.; Gomez, E.D. Ceramic–Salt Composite Electrolytes from Cold Sintering. Adv. Funct. Mater. 2019, 29, 1807872. [Google Scholar] [CrossRef]
- Lowum, S.; Floyd, R.D.; Zhu, Y.; Mao, Z.; Maria, J.-P. Cold sintering of magnetic BaFe12O19 and other ferrites at 300 °C. J. Mater. Sci. 2021, 56, 11229–11236. [Google Scholar] [CrossRef]
- Bouville, F.; Studart, A.R. Geologically-inspired strong bulk ceramics made with water at room temperature. Nat. Commun. 2017, 8, 14655. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamao, N.; Yamaguchi, Y.; Hamamoto, K. Densification of a NASICON-Type LATP Electrolyte Sheet by a Cold-Sintering Process. Materials 2021, 14, 4737. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14164737
Hamao N, Yamaguchi Y, Hamamoto K. Densification of a NASICON-Type LATP Electrolyte Sheet by a Cold-Sintering Process. Materials. 2021; 14(16):4737. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14164737
Chicago/Turabian StyleHamao, Naoki, Yuki Yamaguchi, and Koichi Hamamoto. 2021. "Densification of a NASICON-Type LATP Electrolyte Sheet by a Cold-Sintering Process" Materials 14, no. 16: 4737. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14164737