A PLLA Coating Does Not Affect the Insertion Pressure or Frictional Behavior of a CI Electrode Array at Higher Insertion Speeds
Abstract
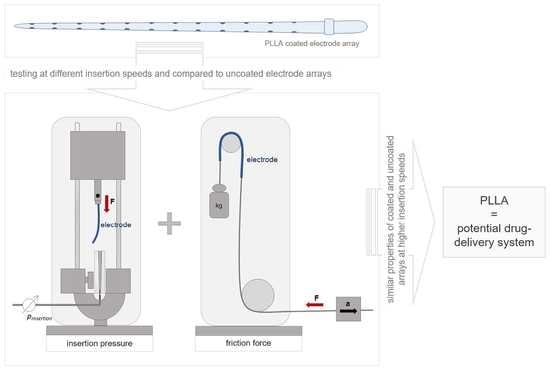
:1. Introduction
2. Materials and Methods
2.1. Electrode Array
2.2. Electrode Array Coating
2.3. Insertion Pressure Measurements and Slope Calculation
2.3.1. Artificial Linear Scala Tympani Model
2.3.2. Insertion Pressure Test Bench
2.3.3. Experimental Procedure
2.3.4. Calculation of the Slope for the Insertion Pressure Curve
2.3.5. Calculation of the Area under the Curve for the Insertion Pressure
2.4. Friction Measurements and Friction Coefficient Calculation
2.4.1. Friction Partner and Lubricant
2.4.2. Friction Test Bench
2.4.3. Experimental Procedure
2.4.4. Friction Coefficient Calculation
2.5. Statistical Analysis
3. Results
3.1. Surface Morphology of Uncoated and PLLA-Coated Electrode Arrays
3.2. Impact of the Insertion Speed on the Electrode Insertion Pressure for Uncoated and Coated Electrode Arrays in the Linear Cochlear Model
3.3. Impact of PLLA Coating on Electrode Insertion Pressure
3.4. Impact of the Moving Speed on the Friction Coefficient of Coated Electrode Arrays
3.5. PLLA Coating Condition after Pressure and Friction Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Image Manipulation
Abbreviations
References
- D’Haese, P.S.C.; Van Rompaey, V.; De Bodt, M.; Van de Heyning, P. Severe Hearing Loss in the Aging Population Poses a Global Public Health Challenge. How Can We Better Realize the Benefits of Cochlear Implantation to Mitigate This Crisis? Front. Public Health 2019, 7, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, R.; Pyman, B.C.; Clark, G.M.; Webb, R.L. Banded Intracochlear Electrode Array: Evaluation of Insertion Trauma in Human Temporal Bones. Ann. Otol. Rhinol. Laryngol. 1985, 94, 55–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tykocinski, M.; Saunders, E.; Cohen, L.T.; Treaba, C.; Briggs, R.J.S.; Gibson, P.; Clark, G.M.; Cowan, R.S.C. The Contour Electrode Array: Safety Study and Initial Patient Trials of a New Perimodiolar Design. Otol. Neurotol. 2001, 22, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, M.; Risi, F.; Escudé, B.; Durmo, I.; James, C.; Lauwers, F.; Deguine, O.; Fraysse, B. Reliability of cone beam computed tomography in scalar localization of the electrode array: A radio histological study. Eur. Arch. Oto-Rhino-Laryngol. 2013, 271, 673–679. [Google Scholar] [CrossRef]
- Wanna, G.B.; Noble, J.H.; Carlson, M.L.; Gifford, R.; Dietrich, M.S.; Haynes, D.S.; Dawant, B.M.; Labadie, R.F. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014, 124, S1–S7. [Google Scholar] [CrossRef]
- Zhou, L.; Friedmann, D.; Treaba, C.; Peng, R.; Roland, J.T., Jr. Does cochleostomy location influence electrode trajectory and intracochlear trauma? Laryngoscope 2014, 125, 966–971. [Google Scholar] [CrossRef]
- Webster, M.; Webster, D.B. Spiral ganglion neuron loss following organ of corti loss: A quantitative study. Brain Res. 1981, 212, 17–30. [Google Scholar] [CrossRef]
- Somdas, M.; Li, P.M.; Whiten, D.M.; Eddington, D.K.; Nadol, J.J.B. Quantitative Evaluation of New Bone and Fibrous Tissue in the Cochlea following Cochlear Implantation in the Human. Audiol. Neurotol. 2007, 12, 277–284. [Google Scholar] [CrossRef]
- Xu, J.; Shepherd, R.; Millard, R.E.; Clark, G.M. Chronic electrical stimulation of the auditory nerve at high stimulus rates: A physiological and histopathological study. Hear. Res. 1997, 105, 1–29. [Google Scholar] [CrossRef]
- Fayad, J.; Linthicum, F.H.; Otto, S.R.; Galey, F.R.; House, W.F. Cochlear Implants: Histopathologic Findings Related to Performance in 16 Human Temporal Bones. Ann. Otol. Rhinol. Laryngol. 1991, 100, 807–811. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Yang, N.W.; Balkany, T.J. Comparative Study of Cochlear Damage With Three Perimodiolar Electrode Designs. Laryngoscope 2003, 113, 415–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardrop, P.; Whinney, D.; Rebscher, S.J.; Roland, J.T., Jr.; Luxford, W.; Leake, P.A. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: Comparison of Nucleus banded and Nucleus ContourTM electrodes. Hear. Res. 2005, 203, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Adunka, O.; Kiefer, J. Impact of electrode insertion depth on intracochlear trauma. Otolaryngol. Neck Surg. 2006, 135, 374–382. [Google Scholar] [CrossRef]
- Gstoettner, W.; Plenk, H.; Franz, P.; Hamzavi, J.; Baumgartner, W.; Czerny, C.; Ehrenberger, K. Cochlear Implant Deep Electrode Insertion: Extent of Insertional Trauma. Acta Oto-Laryngol. 1997, 117, 274–277. [Google Scholar] [CrossRef]
- Ishii, T.; Takayama, M.; Takahashi, Y. Mechanical Properties of Human Round Window, Basilar and Reissner’s Membranes Mechanical Properties of Human Round Window, Basilar and Reissner’s Membranes. Acta Otolaryngol. 1995, 115, 78–82. [Google Scholar] [CrossRef]
- De Seta, D.; Torres, R.; Russo, F.Y.; Ferrary, E.; Kazmitcheff, G.; Heymann, D.; Amiaud, J.; Sterkers, O.; Bernardeschi, D.; Nguyen, Y.; et al. This is a repository copy of Damage to inner ear structure during cochlear implantation: Correlation between insertion force and radio-histological findings in temporal bone specimens. Hear. Res. 2017, 344, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Roland, J.T., Jr. A Model for Cochlear Implant Electrode Insertion and Force Evaluation: Results with a New Electrode Design and Insertion Technique. Laryngoscope 2005, 115, 1325–1339. [Google Scholar] [CrossRef]
- Dohr, D.; Fiedler, N.; Schmidt, W.; Grabow, N.; Mlynski, R.; Schraven, S. Frictional Behavior of Cochlear Electrode Array Is Dictated by Insertion Speed and Impacts Insertion Force. Appl. Sci. 2021, 11, 5162. [Google Scholar] [CrossRef]
- Kontorinis, G.; Lenarz, T.; Stöver, T.; Paasche, G. Impact of the Insertion Speed of Cochlear Implant Electrodes on the Insertion Forces. Otol. Neurotol. 2011, 32, 565–570. [Google Scholar] [CrossRef]
- Zhang, J.; Bhattacharyya, S.; Simaan, N. Model and parameter identification of friction during robotic insertion of cochlear-implant electrode arrays. In Proceedings of the 2009 IEEE International Conference on Robotics and Automation, Kobe, Japan, 12–17 May 2009; pp. 3859–3864. [Google Scholar] [CrossRef]
- Todt, I.; Ernst, A.; Mittmann, P. Effects of Round Window Opening Size and Moisturized Electrodes on Intracochlear Pressure Related to the Insertion of a Cochlear Implant Electrode. Audiol. Neurotol. Extra 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Lehnhardt, E. Intracochlear placement of cochlear implant electrodes in soft surgery technique. HNO 1993, 41, 356–359. [Google Scholar] [PubMed]
- Weiss, N.M.; Dhanasingh, A.; Schraven, S.P.; Schulze, M.; Langner, S.; Mlynski, R. Surgical approach for complete cochlear coverage in EAS-patients after residual hearing loss. PLoS ONE 2019, 14, e0223121. [Google Scholar] [CrossRef] [PubMed]
- Bas, E.; Bohorquez, J.; Goncalves, S.; Perez, E.; Dinh, C.T.; Garnham, C.; Hessler, R.; Eshraghi, A.A.; Van De Water, T.R. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear. Res. 2016, 337, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; O’Leary, S.; Birman, C.; Plant, K.; English, R.; Dawson, P.; Risi, F.; Gavrilis, J.; Needham, K.; Cowan, R.; et al. Comparison of electrode impedance measures between a dexamethasone-eluting and standard CochlearTM Contour Advance® electrode in adult cochlear implant recipients. Hear. Res. 2020, 390, 107924. [Google Scholar] [CrossRef] [PubMed]
- Connolly, T.M.; Eastwood, H.; Kel, G.; Lisnichuk, H.; Richardson, R.; O’Leary, S. Pre-Operative Intravenous Dexamethasone Prevents Auditory Threshold Shift in a Guinea Pig Model of Cochlear Implantation. Audiol. Neurotol. 2011, 16, 137–144. [Google Scholar] [CrossRef]
- James, D.P.; Eastwood, H.; Richardson, R.; O’Leary, S.J. Effects of Round Window Dexamethasone on Residual Hearing in a Guinea Pig Model of Cochlear Implantation. Audiol. Neurotol. 2007, 13, 86–96. [Google Scholar] [CrossRef]
- Lee, J.; Ismail, H.; Lee, J.H.; Kel, G.; O’leary, J.; Hampson, A.; Eastwood, H.; O’leary, S.J. Effect of both local and systemically administered dexamethasone on long-term hearing and tissue response in a guinea pig model of cochlear implantation. Audiol. Neurotol. 2013, 18, 392–405. [Google Scholar] [CrossRef]
- Hadler, C.; Aliuos, P.; Brandes, G.; Warnecke, A.; Bohlmann, J.; Dempwolf, W.; Menzel, H.; Lenarz, T.; Reuter, G.; Wissel, K. Polymer Coatings of Cochlear Implant Electrode Surface—An Option for Improving Electrode-Nerve-Interface by Blocking Fibroblast Overgrowth. PLoS ONE 2016, 11, e0157710. [Google Scholar] [CrossRef] [Green Version]
- Prenzler, N.K.; Salcher, R.; Lenarz, T.; Gaertner, L.; Warnecke, A. Dose-Dependent Transient Decrease of Impedances by Deep Intracochlear Injection of Triamcinolone with a Cochlear Catheter Prior to Cochlear Implantation–1 Year Data. Front. Neurol. 2020, 11, 258. [Google Scholar] [CrossRef]
- Manrique-Huarte, R.; de Linera-Alperi, M.A.; Parilli, D.; Rodriguez, J.; Borro, D.; Dueck, W.; Smyth, D.; Salt, A.; Manrique, M. Inner ear drug delivery through a cochlear implant: Pharmacokinetics in a Macaque experimental model. Hear. Res. 2021, 404, 108228. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kikkawa, Y.S.; Sakamoto, T.; Kondo, K.; Ishihara, K.; Konno, T.; Pawsey, N.; Yamasoba, T. Safety, reliability, and operability of cochlear implant electrode arrays coated with biocompatible polymer. Acta Oto-Laryngol. 2015, 135, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wrzeszcz, A.; Steffens, M.; Balster, S.; Warnecke, A.; Dittrich, B.; Lenarz, T.; Reuter, G. Hydrogel coated and dexamethasone releasing cochlear implants: Quantification of fibrosis in guinea pigs and evaluation of insertion forces in a human cochlea model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Wrzeszcz, A.; Dittrich, B.; Haamann, D.; Aliuos, P.; Klee, D.; Nolte, I.; Lenarz, T.; Reuter, G. Dexamethasone released from cochlear implant coatings combined with a protein repellent hydrogel layer inhibits fibroblast proliferation. J. Biomed. Mater. Res. Part A 2013, 102, 442–454. [Google Scholar] [CrossRef]
- Farahmandghavi, F.; Mirzadeh, H.; Imani, M.; Jolly, C.; Farhadi, M. Corticosteroid-releasing cochlear implant: A novel hybrid of biomaterial and drug delivery system. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 388–398. [Google Scholar] [CrossRef]
- Manrique-Huarte, R.; Zulueta-Santos, C.; Calavia, D.; de Linera-Alperi, M.; Gallego, M.A.; Jolly, C.; Manrique, M. Cochlear Implantation with a Dexamethasone Eluting Electrode Array: Functional and Anatomical Changes in Non-Human Primates. Otol. Neurotol. 2020, 41, e812–e822. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef]
- Wulf, K.; Goblet, M.; Raggl, S.; Teske, M.; Eickner, T.; Lenarz, T.; Grabow, N.; Paasche, G. PLLA Coating of Active Implants for Dual Drug Release. Molecules 2022, 27, 1417. [Google Scholar] [CrossRef]
- Schurzig, D.; Fröhlich, M.; Raggl, S.; Scheper, V.; Lenarz, T.; Rau, T. Uncoiling the Human Cochlea—Physical Scala Tympani Models to Study Pharmacokinetics Inside the Inner Ear. Life 2021, 11, 373. [Google Scholar] [CrossRef]
- Schmidt, W.; Grabow, N.; Behrens, P.; Schmitz, K.-P. Trackability, Crossability, and Pushability of Coronary Stent Systems—An Experimental Approach. Biomed. Eng. Biomed. Tech. 2002, 47 (Suppl. 1), 124–126. [Google Scholar] [CrossRef]
- Meriam, J.L.; Kraige, L.G. Engineering Mechanics: Statics; Wiley Online Library: New York, NY, USA, 1993; pp. 389–390. [Google Scholar]
- Kha, H.; Chen, B. Determination of frictional conditions between electrode array and endosteum lining for use in cochlear implant models. J. Biomech. 2006, 39, 1752–1756. [Google Scholar] [CrossRef]
- Wulf, K.; Teske, M.; Matschegewski, C.; Arbeiter, D.; Bajer, D.; Eickner, T.; Schmitz, K.-P.; Grabow, N. Novel approach for a PTX/VEGF dual drug delivery system in cardiovascular applications—an innovative bulk and surface drug immobilization. Drug Deliv. Transl. Res. 2018, 8, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Kontorinis, G.; Paasche, G.; Lenarz, T.; Stöver, T. The Effect of Different Lubricants on Cochlear Implant Electrode Insertion Forces. Otol. Neurotol. 2011, 32, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Todt, I.; Mittmann, P.; Ernst, A. Intracochlear Fluid Pressure Changes Related to the Insertional Speed of a CI Electrode. BioMed Res. Int. 2014, 2014, 507241. [Google Scholar] [CrossRef] [PubMed]
- Rau, T.S.; Hussong, A.; Leinung, M.; Lenarz, T.; Majdani, O. Automated insertion of preformed cochlear implant electrodes: Evaluation of curling behaviour and insertion forces on an artificial cochlear model. Int. J. Comput. Assist. Radiol. Surg. 2009, 5, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Todd, C.A.; Naghdy, F.; Svehla, M.J. Force Application During Cochlear Implant Insertion: An Analysis for Improvement of Surgeon Technique. IEEE Trans. Biomed. Eng. 2007, 54, 1247–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willard, F.H. Visceral Fascia. In Fascia: The Tensional Network of the Human Body-E-Book: The Science and Clinical Applications in Manual and Movement Therapy; Elsevier Health Sciences: Philadelphia, PA, USA, 2013; p. 54. [Google Scholar]






| Speed (mm/s) | m (Uncoated) (mmhg/mm) | m (Coated) (mmhg/mm) |
|---|---|---|
| 0.1 | 0.11 ± 0.04 | 0.13 ± 0.04 |
| 0.5 | 0.18 ± 0.05 | 0.23 ± 0.05 *** |
| 1.0 | 0.22 ± 0.07 *** | 0.19 ± 0.06 ** |
| 1.5 | 0.18 ± 0.05 | 0.19 ± 0.07 |
| 2.0 | 0.20 ± 0.06 ** | 0.19 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dohr, D.; Wulf, K.; Grabow, N.; Mlynski, R.; Schraven, S.P. A PLLA Coating Does Not Affect the Insertion Pressure or Frictional Behavior of a CI Electrode Array at Higher Insertion Speeds. Materials 2022, 15, 3049. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15093049
Dohr D, Wulf K, Grabow N, Mlynski R, Schraven SP. A PLLA Coating Does Not Affect the Insertion Pressure or Frictional Behavior of a CI Electrode Array at Higher Insertion Speeds. Materials. 2022; 15(9):3049. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15093049
Chicago/Turabian StyleDohr, Dana, Katharina Wulf, Niels Grabow, Robert Mlynski, and Sebastian P. Schraven. 2022. "A PLLA Coating Does Not Affect the Insertion Pressure or Frictional Behavior of a CI Electrode Array at Higher Insertion Speeds" Materials 15, no. 9: 3049. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15093049