Mechanically Robust Dual-Crosslinking Elastomer Enabled by a Facile Self-Crosslinking Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the SCDCEs
2.3. Characterization
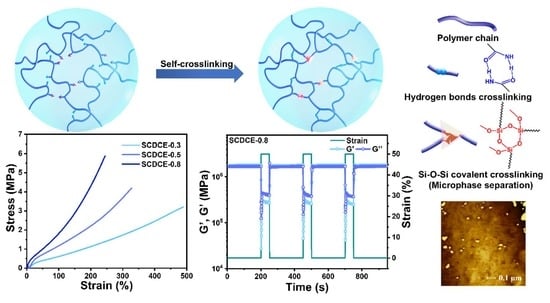
3. Results and Discussion
3.1. Fabrication and Structure
3.2. Dynamic Nature of the Dual Crosslinks
3.3. Mechanical Properties
3.4. Self-Recoverability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Loh, K.J. Wearable Carbon Nanotube-Based Fabric Sensors for Monitoring Human Physiological Performance. Smart Mater. Struct. 2017, 26, 055018. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, S.; Wang, G.-J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly Stretchable Polymer Semiconductor Films through the Nanoconfinement Effect. Science 2017, 355, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, Z.; Hu, H.; Kong, Z.; Chen, C.; Tian, Y.; Zhang, W.; Bin Ying, W.; Zhang, R.; Zhu, J. A Polyurethane Integrating Self-Healing, Anti-Aging and Controlled Degradation for Durable and Eco-Friendly E-Skin. Chem. Eng. J. 2021, 410, 128363. [Google Scholar] [CrossRef]
- Zhou, X.; Gong, Z.; Fan, J.; Chen, Y. Self-Healable, Recyclable, Mechanically Tough Transparent Polysiloxane Elastomers Based on Dynamic Microphase Separation for Flexible Sensor. Polymer 2021, 237, 124357. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, L.; Yang, C.; Yang, Y.; Song, C.; Wu, Q.; Huang, G.; Wu, J. Super Tough and Strong Self-Healing Elastomers Based on Polyampholytes. J. Mater. Chem. A 2018, 6, 19066–19074. [Google Scholar] [CrossRef]
- Matsuda, T.; Nakajima, T.; Gong, J.P. Fabrication of Tough and Stretchable Hybrid Double-Network Elastomers Using Ionic Dissociation of Polyelectrolyte in Nonaqueous Media. Chem. Mater. 2019, 31, 3766–3776. [Google Scholar] [CrossRef]
- Sun, H.; Liu, X.; Yu, B.; Feng, Z.; Ning, N.; Hu, G.-H.; Tian, M.; Zhang, L. Simultaneously Improved Dielectric and Mechanical Properties of Silicone Elastomer by Designing a Dual Crosslinking Network. Polym. Chem. 2019, 10, 633–645. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Q.; Feringa, B.L.; Tian, H.; Qu, D.-H. Toughening a Self-Healable Supramolecular Polymer by Ionic Cluster-Enhanced Iron-Carboxylate Complexes. Angew. Chem. Int. Ed. 2020, 59, 5278–5283. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sun, T.L.; Zhang, A.K.; Ikura, Y.; Nakajima, T.; Nonoyama, T.; Kurokawa, T.; Ito, O.; Ishitobi, H.; Gong, J.P. Tough Physical Double-Network Hydrogels Based on Amphiphilic Triblock Copolymers. Adv. Mater. 2016, 28, 4884–4890. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tang, Z.; Huang, J.; Lin, T.; Guo, B. Strikingly Improved Toughness of Nonpolar Rubber by Incorporating Sacrificial Network at Small Fraction. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 781–786. [Google Scholar] [CrossRef]
- Zhao, X. Designing Toughness and Strength for Soft Materials. Proc. Natl. Acad. Sci. USA. 2017, 114, 8138–8140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, T.; Ozaki, Y.; Namba, R.; Ota, K.; Maida, Y.; Matsuda, T.; Kurokawa, T.; Gong, J.P. Tough Double-Network Gels and Elastomers from the Nonprestretched First Network. ACS Macro Lett. 2019, 8, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, H.; Li, B.; Gao, Y.; Zhao, X.; Zhang, L. Molecular Dynamics Simulation of Fracture Mechanism in the Double Interpenetrated Cross-Linked Polymer. Polymer 2020, 199, 122571. [Google Scholar] [CrossRef]
- Yang, J.; Li, K.; Tang, C.; Liu, Z.; Fan, J.; Qin, G.; Cui, W.; Zhu, L.; Chen, Q. Recent Progress in Double Network Elastomers: One Plus One Is Greater Than Two. Adv. Funct. Mater. 2022, 32, 2110244. [Google Scholar] [CrossRef]
- Luo, M.-C.; Zeng, J.; Fu, X.; Huang, G.; Wu, J. Toughening Diene Elastomers by Strong Hydrogen Bond Interactions. Polymer 2016, 106, 21–28. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, Y.; Wu, Q.; Wang, S.; Huang, G.; Wu, J. Strong and Tough Self-Healing Elastomers Enabled by Dual Reversible Networks Formed by Ionic Interactions and Dynamic Covalent Bonds. Polymer 2018, 157, 172–179. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, Q.; Wei, L.; Fu, X.; Huang, C.; Zhu, Y.; Zhao, L.; Huang, G.; Wu, J. Ultra-Tough, Strong, and Defect-Tolerant Elastomers with Self-Healing and Intelligent-Responsive Abilities. ACS Appl. Mater. Inter. 2019, 11, 29373–29381. [Google Scholar] [CrossRef]
- Parvate, S.; Mahanwar, P. Advances in Self-Crosslinking of Acrylic Emulsion: What We Know and What We Would like to Know. J. Disper. Sci. Technol. 2019, 40, 519–536. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Shen, Q.; Kong, L.; Huang, G.; Wu, J. Tough Underwater Super-Tape Composed of Semi-Interpenetrating Polymer Networks with a Water-Repelling Liquid Surface. ACS Appl. Mater. Inter. 2021, 13, 1535–1544. [Google Scholar] [CrossRef]
- Song, D.; Breedveld, V.; Deng, Y. Rheological Study of Self-Crosslinking and Co-Crosslinking of Ammonium Zirconium Carbonate and Starch in Aqueous Solutions. J. Appl. Polym. Sci. 2011, 122, 1019–1029. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, P.; Li, X.; Xu, Y.; Lu, G.; Jiang, Q.; Sun, Y.; Fan, Y.; Zhang, X. A Di-Self-Crosslinking Hyaluronan-Based Hydrogel Combined with Type I Collagen to Construct a Biomimetic Injectable Cartilage-Filling Scaffold. Acta Biomater. 2020, 111, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Liu, Z.; Lei, Y.; Wang, Y.; Liu, Q.; Yuan, A.; Zhao, Y.; Zhang, X.; Lei, J. Mutually-Complementary Structure Design towards Highly Stretchable Elastomers with Robust Strength and Autonomous Self-Healing Property. Polymer 2020, 186, 122003. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Niu, W.; Yang, X.; Jiang, Z.; Lu, Z.; Liu, X.; Sun, J. Healable and Recyclable Elastomers with Record-High Mechanical Robustness, Unprecedented Crack Tolerance, and Superhigh Elastic Restorability. Adv. Mater. 2021, 33, 2101498. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Fang, X.; Zhang, Z.; Li, S.; Sun, J. Healable and Recyclable Polymeric Materials with High Mechanical Robustness. ACS Mater. Lett. 2022, 4, 554–571. [Google Scholar] [CrossRef]
- Qin, Z.-M.; Wang, P.-C.; Yang, R.; Chen, H.-B. Fast Pyrolysis of Silicones at Low Temperatures Catalyzed by Anatase Titanium Dioxide. Polym. Degrad. Stabil. 2020, 182, 109387. [Google Scholar] [CrossRef]
- Worrell, C.A.; Henshall, T. Vibrational Spectroscopic Studies of Some Lead Silicate Glasses. J. Non-Cryst. Solids 1978, 29, 283–299. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, T.; Jiang, X.; Liu, X.; Shi, D.; Liu, C.; Yang, S.; Chen, E.-Q. Thermoplastic High Strain Multishape Memory Polymer: Side-Chain Polynorbornene with Columnar Liquid Crystalline Phase. Adv. Mater. 2017, 29, 1605908. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sun, Y.; Wang, M.; Wu, B.; Han, G.; Jin, L.; Zhang, K.; Xia, Y. Self-Healable and Reprocessable Acrylate-Based Elastomers with Exchangeable Disulfide Crosslinks by Thiol-Ene Click Chemistry. Polymer 2021, 212, 123132. [Google Scholar] [CrossRef]
- Lan, J.; Li, Y.; Yan, B.; Yin, C.; Ran, R.; Shi, L.-Y. Transparent Stretchable Dual-Network Ionogel with Temperature Tolerance for High-Performance Flexible Strain Sensors. ACS Appl. Mater. Inter. 2020, 12, 37597–37606. [Google Scholar] [CrossRef]
- Nomimura, S.; Osaki, M.; Park, J.; Ikura, R.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-Healing Alkyl Acrylate-Based Supramolecular Elastomers Cross-Linked via Host–Guest Interactions. Macromolecules 2019, 52, 2659–2668. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Wang, M.; Wang, Z.; Han, G.; Jin, L.; Lin, P.; Xia, Y.; Zhang, K. Mechanically Robust and Reprocessable Acrylate Vitrimers with Hydrogen-Bond-Integrated Networks for Photo-3D Printing. ACS Appl. Mater. Inter. 2021, 13, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Geng, Y.; Cao, H.; Zhou, J.; Tian, Y.; Shan, G.; Bao, Y.; Wu, Z.L.; Pan, P. Dual-Crosslink Physical Hydrogels with High Toughness Based on Synergistic Hydrogen Bonding and Hydrophobic Interactions. Macromol. Rapid Commun. 2018, 39, 1700806. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Mai, Y.; Li, W.; Liao, B. Supersoft, Stretchable, Tough, and Adhesive Elastomers with Dual Metal-Ionic Crosslinked Double-Network Structure. Macromol. Chem. Phys. 2020, 221, 1900516. [Google Scholar] [CrossRef]
- Hou, J.; Ren, X.; Guan, S.; Duan, L.; Gao, G.H.; Kuai, Y.; Zhang, H. Rapidly Recoverable, Anti-Fatigue, Super-Tough Double-Network Hydrogels Reinforced by Macromolecular Microspheres. Soft Matter 2017, 13, 1357–1363. [Google Scholar] [CrossRef]
- Xiong, H.; Zhang, L.; Wu, Q.; Zhang, H.; Peng, Y.; Zhao, L.; Huang, G.; Wu, J. A Strain-Adaptive, Self-Healing, Breathable and Perceptive Bottle-Brush Material Inspired by Skin. J. Mater. Chem. A 2020, 8, 24645–24654. [Google Scholar] [CrossRef]
- Wu, Q.; Xiong, H.; Peng, Y.; Yang, Y.; Kang, J.; Huang, G.; Ren, X.; Wu, J. Highly Stretchable and Self-Healing “Solid–Liquid” Elastomer with Strain-Rate Sensing Capability. ACS Appl. Mater. Inter. 2019, 11, 19534–19540. [Google Scholar] [CrossRef]
- Bueche, F. Mullins Effect and Rubber–Filler Interaction. J. Appl. Polym. Sci. 1961, 5, 271–281. [Google Scholar] [CrossRef]
- Ogden, R.W.; Roxburgh, D.G. A Pseudo–Elastic Model for the Mullins Effect in Filled Rubber. Proc. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 1999, 455, 2861–2877. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.; Jin, B.; Wu, H.; Zeng, Z.; Huang, M.; Wu, J.; Liao, L.; Zheng, J. Mechanically Robust Dual-Crosslinking Elastomer Enabled by a Facile Self-Crosslinking Approach. Materials 2022, 15, 3983. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15113983
Huang Z, Jin B, Wu H, Zeng Z, Huang M, Wu J, Liao L, Zheng J. Mechanically Robust Dual-Crosslinking Elastomer Enabled by a Facile Self-Crosslinking Approach. Materials. 2022; 15(11):3983. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15113983
Chicago/Turabian StyleHuang, Zhendong, Biqiang Jin, Haitao Wu, Zihang Zeng, Minghui Huang, Jinrong Wu, Lusheng Liao, and Jing Zheng. 2022. "Mechanically Robust Dual-Crosslinking Elastomer Enabled by a Facile Self-Crosslinking Approach" Materials 15, no. 11: 3983. https://0-doi-org.brum.beds.ac.uk/10.3390/ma15113983