Lipoproteins as Drug Carriers for Cyclosporine A: Optimization of the Entrapment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Plasma or Lipoproteins
2.2.2. Incubation of Cyclosporine A with Plasma or Lipoproteins
2.2.3. Protein and Lipid Analyses
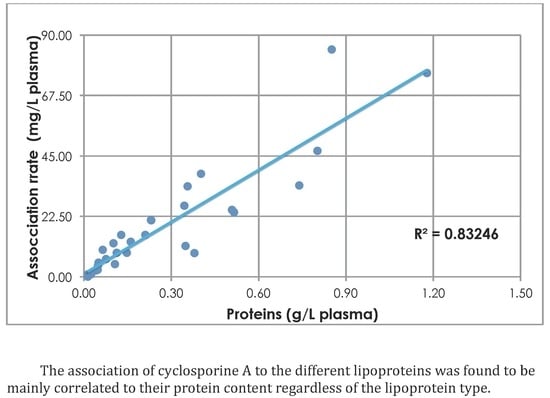
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kockx, M.; Kritharides, L. Cyclosporin A-Induced Hyperlipidemia. In Lipoproteins—Role in Health and Diseases; Kostner, G., Ed.; InTech: Rang-Du-Fliers, Paris, 2012; Available online: http://www.intechopen.com/books/lipoproteins-role-in-health-and-diseases/cyclosporin-a-induced-hyperlipidemia (accessed on 1 June 2020).
- Akhlaghi, F.; Trull, A.K. Distribution of Cyclosporin in Organ Transplant Recipients. Clin. Pharmacokinet. 2002, 41, 615–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Huang, L.; Chu, I. Controlled Release of Cyclosporine A from Biodegradable Amphiphilic Diblock Copolymer Sol-gel Drug Delivery System. J. Med. Biol. Eng. 2011, 31, 177. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A. Nanoparticles for Treatment of Atopic Dermatitis. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 167–175. Available online: https://0-linkinghub-elsevier-com.brum.beds.ac.uk/retrieve/pii/B9780128029268000136 (accessed on 5 September 2019).
- Abdel-Mottaleb, M.M.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine strategies for targeting skin inflammation. Nanomedicine 2014, 9, 1727–1743. [Google Scholar] [CrossRef]
- Wasan, K.M.; Ramaswamy, M.; Kwong, M.; Boulanger, K.D. Role of plasma lipoproteins in modifying the toxic effects of water-insoluble drugs: Studies with cyclosporine A. AAPS PharmSci. 2002, 4, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, G.; Chen, J.; Li, H.; Glickson, J.D. Rerouting lipoprotein nanoparticles to selected alternate receptors for the targeted delivery of cancer diagnostic and therapeutic agents. Proc. Natl. Acad. Sci. USA 2005, 102, 17757–17762. [Google Scholar] [CrossRef] [Green Version]
- Harisa, G.I.; Alanazi, F.K. Low density lipoprotein bionanoparticles: From cholesterol transport to delivery of anti-cancer drugs. Saudi Pharm. J. 2014, 22, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Scarcello, E.; Abdel-Mottaleb, M.M.A.; Beduneau, A.; Moulari, B.; Pellequer, Y. Amelioration of murine experimental colitis using biocompatible cyclosporine A lipid carriers. Drug Deliv. Transl. Res. 2021, 11, 1301–1308. Available online: http://0-link-springer-com.brum.beds.ac.uk/10.1007/s13346-020-00835-z (accessed on 20 October 2020). [CrossRef]
- Bijsterbosch, M.K.; van Berkel, T.J.C. Native and modified lipoproteins as drug delivery systems. Adv. Drug Deliv. Rev. 1990, 5, 231–251. [Google Scholar] [CrossRef]
- Firestone, R.A. Low-Density Lipoprotein as a Vehicle for Targeting Antitumor Compounds to Cancer Cells. Bioconjugate Chem. 1994, 5, 105–113. [Google Scholar] [CrossRef]
- Gerster, R.; Eloranta, J.J.; Hausmann, M.; Ruiz, P.A.; Cosin-Roger, J.; Terhalle, A.; Ziegler, U.; Kullak-Ublick, G.; von Eckardstein, A.; Rogler, G. Anti-inflammatory Function of High-Density Lipoproteins via Autophagy of IκB Kinase. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 171–187.e1. [Google Scholar] [CrossRef]
- Carlson, K. Lipoprotein fractionation. J. Clin. Pathol. 1973, 5, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautier, T.; Masson, D.; de Barros, J.-P.P.; Athias, A.; Gambert, P.; Aunis, D.; Metz-Boutigue, M.-H.; Lagrost, L. Human Apolipoprotein C-I Accounts for the Ability of Plasma High Density Lipoproteins to Inhibit the Cholesteryl Ester Transfer Protein Activity. J. Biol. Chem. 2000, 275, 37504–37509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Chao, F.-F.; Pham, Q.; Thiessen, J.; Soldin, S.J. The role of lipoproteins in the transport and uptake of cyclosporine and dihydro-tacrolimus into HepG2 and JURKAT cell lines. Clin. Biochem. 1996, 29, 149–155. [Google Scholar] [CrossRef]
- Sgoutas, D.; Macmahon, W.; Love, A.; Jerkunica, I. Interaction of cyclosporin A with human lipoproteins. J. Pharm. Pharmacol. 1986, 38, 583–588. [Google Scholar] [CrossRef]
- Prueksaritanont, T.; Koike, M.; Hoener, B.-A.; Benet, L.Z. Transport and metabolism of cyclosporine in isolated rat hepatocytes: The effects of lipids. Biochem. Pharmacol. 1992, 43, 1997–2006. [Google Scholar] [CrossRef]
- Strong, M.L.; Ueda, C.T. Effects of Low and High Density Lipoproteins on Renal Cyclosporine A and Cyclosporine G Disposition in the Isolated Perfused Rat Kidney. Pharm. Res. 1997, 14, 1466–1471. [Google Scholar] [CrossRef]
- Wasan, K.M.; Haydn Pritchard, P.; Rama, M.; Wong, W.; Donnachie, E.; Brunner, L. Differences in Lipoprotein Lipid Concentration and Composition Modify the Plasma Distribution of Cyclosporine. Pharm. Res. 1997, 14, 1613–1620. [Google Scholar] [CrossRef]
- De Klippel, N.; Sennesael, J.; Lamote, J.; Ebinger, G.; Dekeyser, J. Cyclosporin leukoencephalopathy induced by intravenous lipid solution. Lancet 1992, 339, 1114. [Google Scholar] [CrossRef]
- Shibata, N.; Shimakawa, H.; Minouchi, T.; Yamaji, A. Erythrocyte Uptake and Protein Binding of Cyclosporin A (CyA) in Human Blood: Factors Affecting CyA Concentration in Erythrocytes. Biol. Pharm. Bull. 1993, 16, 702–707. [Google Scholar] [CrossRef]
- Rödl, S.; Fuchs, G.; Khoshsorur, G.; Iberer, F.; Tscheliessnigg, K.H. Lipoprotein-induced modulation of cyclosporine-A-mediated immunosuppression. Eur. J. Clin. Investig. 1990, 20, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, F.; McLachlan, A.J.; Keogh, A.M.; Brown, K.F. Effect of simvastatin on cyclosporine unbound fraction and apparent blood clearance in heart transplant recipients. Br. J. Clin. Pharmacol. 1997, 44, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groen, P.C. Cyclosporine, Low-Density Lipoprotein, and Cholesterol. Mayo Clin. Proc. 1988, 63, 1012–1021. [Google Scholar] [CrossRef]
- Gardier, A.; Mathe, D.; Guedeney, X.; Barre, J.; Benvenutti, C.; Navarro, N.; Vernillet, L.; Loisance, D.; Cachera, J.P.; Jacotot, B.; et al. Effects of plasma lipid levels on blood distribution and pharmacokinetics of cyclosporin A. Ther. Drug Monit. 1993, 15, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lichtiger, S. The role of cyclosporine therapy in ulcerative colitis treatment. Gastroenterol. Hepatol. 2006, 9, 624–626. [Google Scholar]
- Loftus, C.G.; Loftus, E.V., Jr.; Sandborn, W.J. Cyclosporin for refractory ulcerative colitis. Gut 2003, 52, 172–173. [Google Scholar] [CrossRef] [Green Version]
- Sethi, P.; White, C.; Cummings, B.; Hines, R.N.; Muralidhara, S.; Bruckner, J.V. Ontogeny of plasma proteins, albumin and binding of diazepam, cyclosporine, and deltamethrin. Pediatr Res. 2016, 79, 409–415. [Google Scholar] [CrossRef]




| Time (min) | ACN (%) | H2O (%) |
|---|---|---|
| 0 | 68 | 32 |
| 2 | 68 | 32 |
| 5 | 85 | 15 |
| 5.1 | 95 | 5 |
| 7.1 | 95 | 5 |
| 7.2 | 68 | 32 |
| 10.2 | 68 | 32 |
| Lipoprotein | Correlation Coefficient Between LPs’ Different Components and Association Efficiency | |||||
|---|---|---|---|---|---|---|
| TG | PL | TC | FC | Proteins | EC | |
| HDL | 0.7513 | 0.1196 | 0.5134 | 0.5610 | 0.8138 | 0.4889 |
| LDL | 0.3721 | 0.2337 | 0.3392 | 0.3079 | 0.8090 | 0.3386 |
| VLDL | 0.9325 | 0.6459 | 0.9830 | 0.8854 | 0.9608 | 0.9875 |
| Carrier | Type of Lipoproteins | TG (g/L Plasma) | PL (g/L Plasma) | TC (g/L Plasma) | FC (g/L Plasma) | Proteins (g/L Plasma) | EC (g/L Plasma) | Encapsulation Rate (mg/L Plasma) |
|---|---|---|---|---|---|---|---|---|
| isolated LPs | HDL | 0.120 | 0.160 | 0.080 | 0.01 | 0.342 | 0.070 | 27.700 |
| LDL | 0.970 | 0.370 | 0.52 | 0.12 | 0.566 | 0.400 | 29.100 | |
| VLDL | 2.890 | 0.310 | 0.420 | 0.15 | 0.572 | 0.270 | 45.000 | |
| full Plasma | HDL | 0.290 | 0.230 | 0.150 | 0.04 | 0.673 | 0.110 | 22.200 |
| LDL | 1.620 | 0.360 | 0.630 | 0.14 | 0.774 | 0.490 | 17.200 | |
| VLDL | 3.800 | 0.250 | 0.470 | 0.16 | 0.741 | 0.310 | 31.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Mottaleb, M.M.A.; Boi, L.; Barra, M.; Colin, J.; Berni, L.; Béduneau, A.; Moulari, B.; Pellequer, Y. Lipoproteins as Drug Carriers for Cyclosporine A: Optimization of the Entrapment. Materials 2023, 16, 1156. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16031156
Abdel-Mottaleb MMA, Boi L, Barra M, Colin J, Berni L, Béduneau A, Moulari B, Pellequer Y. Lipoproteins as Drug Carriers for Cyclosporine A: Optimization of the Entrapment. Materials. 2023; 16(3):1156. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16031156
Chicago/Turabian StyleAbdel-Mottaleb, Mona M. A., Lorenza Boi, Marina Barra, Julie Colin, Luisa Berni, Arnaud Béduneau, Brice Moulari, and Yann Pellequer. 2023. "Lipoproteins as Drug Carriers for Cyclosporine A: Optimization of the Entrapment" Materials 16, no. 3: 1156. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16031156