Proactive Effect of Algae-Based Graphene Support on the Oxygen Evolution Reaction Electrocatalytic Activity of NiFe
Abstract
:1. Introduction
2. Materials and Methods
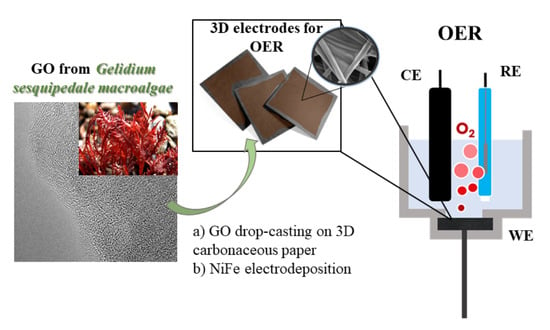
2.1. Preparation of Graphene Oxide-like Materials from Algae
2.2. Preparation of 3D Electrodes
2.3. Characterization of Graphene Materials and 3D Electrodes
2.4. Electrochemical Characterization
3. Results and Discussion
3.1. Preparation of Graphene Materials from Algae Waste
3.2. Synthesis, Characterization, and Evaluation of the Catalytic Activity of Algae-Based and Graphite-Based 3D Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alfani, D.; Binotti, M.; Macchi, E.; Silva, P.; Astolfis, M. CO2 power plants for waste heat recovery: Design optimization and part-load operation strategies. Appl. Therm. Eng. 2021, 195, 117013. [Google Scholar] [CrossRef]
- Huang, H.; Liu, M.; Li, X.; Guo, X.; Wang, T.; Li, S.; Lei, H. Numerical simulation and visualization study of a new tapered-slope serpentine flow field in proton exchange membrane fuel cell. Energy 2022, 246, 123406. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of Electrocatalysts for Oxygen- and Hy-drogen-Involving Energy Conversion Reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Shao, Q.; Huang, X. Recent Progress in Advanced Electrocatalyst Design for Acidic Oxygen Evolution Reaction. Adv. Mater. 2021, 33, 2004243. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, F.; Zeng, Z.; Sinev, I.; Mezdorf, T.; Deshpande, S.; Bernal Lopez, M.; Kunze, S.; Zegkinoglou, I.; Sarodnik, H.; Fan, D.; et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 2020, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.L.; Godwin, I.J.; Brandon, M.P.; Lyons, M.E.G. Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes. Phys. Chem. Chem. Phys. 2013, 15, 13737–13783. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Dai, H. A mini review on NiFe-based materials as highly active oxygen evolution reaction electrocatalysts. Nano Res. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Wang, Q.; Shang, L.; Shi, R.; Zhang, X.; Zhao, Y.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Zhang, T. NiFe layered double hydroxide nanoparticles on Co,N-codoped carbon nanoframes as efficient bifunctional catalysts for rechargeable Zinc-Air batteries. Adv. Energy Mater. 2017, 7, 1700467. [Google Scholar] [CrossRef]
- Meng, C.; Ling, T.; Ma, T.-Y.; Wang, H.; Hu, Z.; Zhou, Y.; Mao, J.; Du, X.-W.; Jaroniec, M.; Qiao, S.-Z. Atomically and Electronically Couples Pt and CoO Hybrid nanocatalysts for Enhanced Electrocatalytic Performance. Adv. Mater. 2017, 29, 1604607. [Google Scholar] [CrossRef]
- Tahir, M.; Mahmood, N.; Zhu, J.; Mahmood, A.; Butt, F.K.; Rizwan, S.; Aslam, I.; Tanveer, M.; Idrees, F.; Shakir, I.; et al. One Dimensional Graphitic Carbon Nitrides as effective Metal-Free Oxygen Reduction Catalysts. Sci. Rep. 2015, 5, 12389. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.; Wang, H.; Liang, Y.; Wu, J.Z.; Zhou, J.; Wang, J.; Regier, T.; Wei, F.; Dai, H. An Advanced Ni-Fe Layered Double Hydroxide Electrocatalyst for Water Oxidation. J. Am. Chem. Soc. 2013, 135, 8452–8455. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z. Hydrogen evolution: Guiding principles. Nat. Energy 2016, 1, 16155. [Google Scholar] [CrossRef]
- Tahir, M.; Pan, L.; Idrees, F.; Zhang, X.; Wang, L.; Zou, J.; Wang, Z.L. Electrocatalytic oxygen evolution reaction for energy conversion and storage: A comprehensive review. Nano Energy 2017, 37, 136–157. [Google Scholar] [CrossRef]
- Jia, Y.; Yao, X. Defects in Carbon-Based Materials for Electrocatalysis. Acc. Chem. Res. 2023, 56, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Li, N.; Bao, L.; Zhang, P.; Lu, X. Intrinsic carbon structural imperfections for enhancing energy conversion electrocatalysts. Chem. Eng. J. 2023, 466, 143060. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Du, D.; Lin, Y. Robust noble metal-based electrocatalysts for oxygen evolution reaction. Chem. Soc. Rev. 2019, 48, 3181–3192. [Google Scholar] [CrossRef] [PubMed]
- Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaría, R.; Menéndez, R. New alternatives to graphite for producing Graphene materials. Carbon 2015, 193, 812–818. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.; Chuah, J.; Vo, D.; Ma, N.; Lam, W.; Lam, S. Transformation of biomass into carbon nanofiber for supercapacitor application—A review. Int. J. Hydrogen Enery 2018, 48, 20811–20821. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of recent advances in thermos-chemical conversion of biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Athanasiou, M.; Yannopoulos, S.; Ioannides, T. Biomass-derived graphene-like materials as active electrodes for supercapacitor applications: A critical review. Chem. Eng. J. 2022, 446, 137191. [Google Scholar] [CrossRef]
- Ferrera-Lorenzo, N.; Fuente, E.; Bermúdez, J.M.; Suárez-Ruiz, I.; Ruiz, B. Conventional and microwave pyrolysis of a macroalgae waste from the Agar-Agar industry. Prospects for bio-fuel production. Bioresour. Technol. 2014, 151, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Cassiani-Cassiani, D.; Meza-González, D.A.; González-Delgado, A.D. Environmental Evaluation of Agar Production from Macroalgae Gracilaria sp. Chem. Eng. Trans. 2018, 70, 2005–2010. [Google Scholar] [CrossRef]
- Lopez-Anton, M.A.; Ferrera-Lorenzo, N.; Fuente, E.; Díaz-Somoano, M.; Suarez-Ruíz, I.; Martínez-Tarazona, M.R.; Ruiz, B. Impact of oxy-fuel combustion gases on mercury retention in activated carbons from a macroalgae waste: Effect of wáter. Chemosphere 2015, 125, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ferrera-Lorenzo, N.; Fuente, E.; Suárez-Ruiz, I.; Ruiz, B. Sustainable activated carbons of macroalgae waste from the Agar-Agar industry. Prospects as adsorbent for gas storage at high pressures. Chem. Eng. J. 2014, 250, 128–136. [Google Scholar] [CrossRef]
- Ferrera-Lorenzo, N.; Fuente, E.; Suárez-Ruiz, I.; Ruiz, B. KOH activated carbon from conventional and microwave heating system of a macroalgae waste from the Agar-Agar industry. Fuel Process. Technol. 2014, 121, 25–31. [Google Scholar] [CrossRef]
- Sanchez-Page, B.; Pérez-Mas, A.; González-Ingelmo, M.; Fernández, L.; González, Z.; Jiménez, M.V.; Pérez-Torrente, J.J.; Blasco, J.; Subías, G.; Álvarez, P.; et al. Influence of graphene sheet properties as supports of iridium-based N-heterocyclic carbene hybrid materials for water oxidation electrocatalysis. J. Organomet. Chem. 2020, 919, 121334. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structure three-dimensional nickel-iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, P.M.A. Practical Surface Analysis in Auger and X-ray Photoelectron Spectroscopy, 2nd ed.; Briggs, D., Seah, M.P., Eds.; Wiley: New York, NY, USA, 1990; Volume 1, p. 657. [Google Scholar]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Deng, D.; Pan, X.; Yu, L.; Cui, Y.; Jiang, Y.; Qi, J.; Li, W.-X.; Fu, Q.; Ma, X.; Xue, Q.; et al. Toward N-Doped Graphene via Solvothermal Synthesis. Chem. Mater. 2011, 23, 1188–1193. [Google Scholar] [CrossRef]
- Díez Betriu, X.; Álvarez García, S.; Botas, C.; Álvarez, P.; Sánchez Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman spectroscopy for the study of reduction mechanisms and optimization of conductivity in graphene oxide thin films. J. Mater. Chem. C 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Romano, V.; Torrisi, L.; Cutroneo, M.; Havranek, V.; Angelo, G.D. Raman investigation of laser-induced structural defects of graphite oxide films. EPJ Web Conf. 2018, 167, 1–5. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. The Raman fingerprint of graphene. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Bykkam, S.; Rao, V.; Chakra, C.H.C.; Thunugunta, T. Synthesis and characterization of graphene oxide and its antimicrobial activity against klebseilla and staphylococus. Int. J. Adv. Biotechnol. Res. 2013, 4, 142–146. [Google Scholar]
- Somanathan, T.; Prasad, K.; Ostrikov, K.; Saravanan, A.; Krishna, V. Graphene oxide synthesis from Agro Waste. Nanomaterials 2015, 5, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Kusurkar, T.S.; Maurya, S.K.; Meena, S.K.; Singh, S.K.; Sethy, N.; Bhargava, K.; Sharma, R.K.; Goswami, D.; Sarkar, S.; et al. Graphene oxide from silk cocoon: A novel magnetic fluorophore for multi-photon imaging. 3 Biotech 2014, 4, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Shuai, X.; Mao, S.; Yang, H.; Qian, J.; Chen, J.; Yan, J.; Cen, K. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 2014, 4, 4684. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Kumar, M.; Sasikumar, M.; Arulraj, A.; Rajasudha, V.; Murugadoss, G.; Kumar, M.R.; Peera, S.G.; Mangalaraja, R.V. NiFe Layered Double Hydroxide Electrocatalyst Prepared via an Electrochemical Deposition Method for the Oxygen Evolution Reaction. Catalysts 2022, 12, 1470. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Hsu, C.; Kuo, D. Activating nickel iron layer double hydroxide for alkaline hydrogen evolution reaction and overall water splitting by electrodepositing nickel hydroxide. Chem. Eng. J. 2021, 419, 129608. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.M.; Yoon, S.; Muller, C.R. Dry-reforming of methane over bimetallic Ni–M/La2O3 (M = Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, P.; Qin, Z.; Yu, C.; Li, W.; Qin, Q.; Li, B.; Fan, M.; Liang, X.; Dong, L. Low temperature CO oxidation catalysed by flower-like Ni–Co–O: How physicochemical properties influence catalytic performance. RSC Adv. 2018, 8, 7110. [Google Scholar] [CrossRef] [PubMed]
- Kosova, N.V.; Devyatkina, E.T.; Kaichev, V.V. Mixed layered Ni–Mn–Co hydroxides: Crystal structure, electronic state of ions, and thermal decomposition. J. Power Sources 2007, 174, 735–740. [Google Scholar] [CrossRef]
- Salagre, P.; Fierro, J.L.G.; Medina, F.; Sueiras, J.E. Characterization of nickel species on several g-alumina supported nicke samples. J. Mol. Catal. A Chem. 1996, 106, 125–134. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, H.; Chen, X.; Liu, H.; Zhao, Y.; Li, H.; Xie, W.; Cheng, F. Anion insertion enhanced electrodeposition of robust metal hydroxide/oxide electrodes for oxygen evolution. Nat. Commun. 2018, 9, 2373. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Guo, M.; Zhang, Q. Scalable electrodeposition of NiFe-based electrocatalysts with self-evolving multi-vacancies for high-performance industrial water electrolysis. Appl. Catal. B 2023, 322, 122101. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Pan, Q.; Ba, J.; Tang, T.; Luo, W. One-Step Synthesis of NiFe Layered Double Hydroxide Nanosheet Array/N-Doped Graphite Foam Electrodes for Oxygen Evolution Reactions. ChemistryOpen 2019, 8, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Gao, H.Y.; Xing, L.W.; Chen, X.; Dong, W.J.; Huang, X.B.; Wang, G. 3D Self-Supported Porous NiO@NiMoO4 Core–Shell Nanosheets for Highly Efficient Oxygen Evolution Reaction. Inorg. Chem. 2019, 58, 6758–6764. [Google Scholar] [CrossRef]









| Sample | C (%) | O (%) | N (%) | Mg (%) | Ca (%) | Na (%) | S (%) | P (%) | K (%) |
|---|---|---|---|---|---|---|---|---|---|
| Al | 72.4 | 18.2 | 1.0 | 0.4 | 3.4 | 0.5 | 1.3 | 0.7 | 1.9 |
| Al-GO | 72.3 | 26.4 | 1.1 | - | - | - | 0.3 | - | - |
| Al-GO-400 | 84.8 | 13.7 | 1.5 | - | - | - | - | - | - |
| G-GO | 65.8 | 34.2 | - | - | - | - | - | - | - |
| G-GO-400 | 85.9 | 14.1 | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Ingelmo, M.; Granda, M.; Ruiz, B.; Fuente, E.; Sierra, U.; Rocha, V.G.; González, Z.; Álvarez, P.; Menéndez, R. Proactive Effect of Algae-Based Graphene Support on the Oxygen Evolution Reaction Electrocatalytic Activity of NiFe. Materials 2023, 16, 7641. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16247641
González-Ingelmo M, Granda M, Ruiz B, Fuente E, Sierra U, Rocha VG, González Z, Álvarez P, Menéndez R. Proactive Effect of Algae-Based Graphene Support on the Oxygen Evolution Reaction Electrocatalytic Activity of NiFe. Materials. 2023; 16(24):7641. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16247641
Chicago/Turabian StyleGonzález-Ingelmo, María, Marcos Granda, Begoña Ruiz, Enrique Fuente, Uriel Sierra, Victoria G. Rocha, Zoraida González, Patricia Álvarez, and Rosa Menéndez. 2023. "Proactive Effect of Algae-Based Graphene Support on the Oxygen Evolution Reaction Electrocatalytic Activity of NiFe" Materials 16, no. 24: 7641. https://0-doi-org.brum.beds.ac.uk/10.3390/ma16247641