Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussion
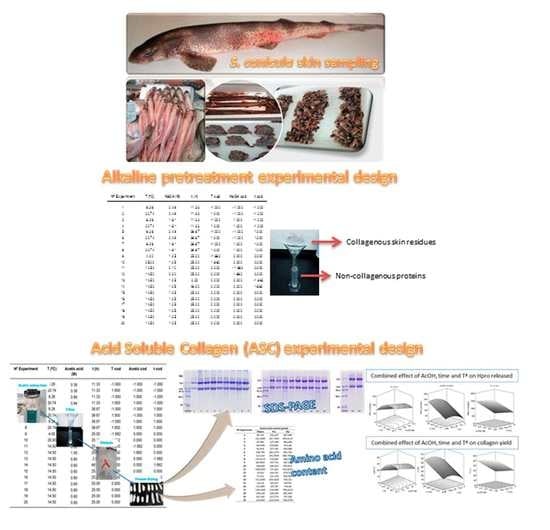
2.1. Alkaline Pre-Treatment of Skin
2.2. Acid-Soluble Collagen (ASC) Extraction Stage
3. Material and Methods
3.1. Biological Samples and Compositional Analysis
3.2. Experimental Design and Statistical Analysis
3.2.1. Alkaline Pre-Treatment Experimental Design
3.2.2. Acid-Soluble Collagen Extraction Stage Experimental Design
3.2.3. Mathematical Modelling and Statistical Analysis
3.3. Amino Acid Characterization of Acid-Soluble Collagen
3.4. SDS-PAGE Characterization of Acid-Soluble Collagen
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blanco, M. Valorización de Descartes y Subproductos de Pintarroja (Scyliorhinus canicula). Ph.D. Thesis, Universidad de Vigo, Vigo, Spain, 2015. [Google Scholar]
- Regulation (EU) No 1380/2013 of the European Parliament and of the Council of the European Union. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:354:0022:0061:EN:PDF (accessed on 29 November 2018).
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjakul, S.; Nalinanon, S.; Shahidi, F. Fish collagen. In Food Biochemistry and Food Processing, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2012; pp. 365–387. [Google Scholar]
- Alves, A.L.; Marques, A.L.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ramos-Ariza, P.; Pérez-Martín, R.I. Characterization of collagen from different discarded fish species of the west coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2016, 25, 388–399. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine Fish proteins and Peptides from Cosmoceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-W.; Dong, X.-P.; Zhou, D.-Y.; Gao, Y.; Yang, J.-F.; Li, D.-M.; Zhao, X.-K.; Ren, T.-T.; Ye, W.-X.; Tan, H.; et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocoll. 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Kellerman, J.; Müller, W.E.; Kreuter, J. Marine sponge collagen: Isolation, characterization and effects on the skin parameters surface-pH, moisture and sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Shen, X.R.; Kurihara, H.; Takahashi, K. Characterization of molecular species of collagen in scallop mantle. Food Chem. 2007, 102, 1187–1191. [Google Scholar] [Green Version]
- Liu, D.; Wei, G.; Li, T.; Hu, J.; Lu, N.; Regenstein, J.M.; Zhou, P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015, 172, 836–843. [Google Scholar] [CrossRef]
- Woo, J.W.; Yu, S.J.; Cho, S.M.; Lee, Y.B.; Kim, S.B. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocoll. 2008, 22, 879–887. [Google Scholar] [CrossRef]
- Wang, L.; Yang, B.; Du, X.; Yang, Y.; Liu, J. Optimization of conditions for extraction of acid-soluble collagen from grass carp (Ctenopharyngodon idella) by response surface methodology. Innov. Food Sci. Emerg. Technol. 2008, 9, 604–607. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterization of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Benjakul, S.; Thiansilakul, Y.; Visessanguan, W.; Roytrakul, S.; Kishimura, H.; Prodpran, T.; Meesane, J. Extraction and characterisation of pepsin-solubilised collagen from the skin of bigeye snapper (Priacanthus tayenus and Priacanthusmacracanthus). J. Sci. Food Agric. 2010, 90, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterization of pepsin-solubilized collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalamichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and characterization of collagen from the skin of brownbanded bamboo shark. Food Chem. 2010, 119, 1519–1526. [Google Scholar] [CrossRef]
- Blanco, M.; Fraguas, J.; Sotelo, C.G.; Pérez-Martín, R.I.; Vázquez, J.A. Production of chondroitin sulphate from head, skeleton and fins of Scyliorhinus canicula by-products by combination of enzymatic, chemical precipitation and ultrafiltration methodologies. Mar. Drugs 2015, 13, 3289–3308. [Google Scholar] [CrossRef] [PubMed]
- Murado, M.A.; Montemayor, M.I.; Cabo, M.L.; Vázquez, J.A.; González, M.P. Optimization of extraction and purification process of hyaluronic acid from fish eyeball. Food Bioprod. Process. 2012, 90, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.A.; O´Brien, W.D., Jr. Modified assay for determination of hydroxyproline in a tissue hydrolysate. Clin. Chim. Acta 1980, 104, 161–167. [Google Scholar] [CrossRef]
- Eastoe, J.; Eastoe, B. A method for the determination of total nitrogen in proteins. Br. Gel. Glue Res. Assoc. Res. Rep. 1952, 5, 1–17. [Google Scholar]
- Zhou, P.; Regenstein, J.M. Effects of alkaline and acid pretreatments on Alaska Pollock skin gelatin extraction. J. Food Sci. 2005, 70, C392–C396. [Google Scholar] [CrossRef]
- Regenstein, J.M.; Zhou, P. Collagen and gelation from marine by-product. In Maximizing the Value of Marine by-Products, 1st ed.; Shahidi, F., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 279–303. [Google Scholar]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Senaratne, S.S.; Park, P.J.; Kim, S.K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour. Technol. 2006, 97, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Shahidi, F. Isolation and properties of acid and pepsin-soluble collagen from the skin of blacktip shark (Carcharhinus limbatus). Eur. Food. Res. Technol. 2010, 230, 475–483. [Google Scholar] [CrossRef]
- Bae, I.; Osatomi, K.; Yoshida, A.; Osako, K.; Yamaguchi, A.; Hara, K. Biochemical properties of acid-soluble collagens extracted from the skin of underutilised fishes. Food Chem. 2008, 108, 49–54. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemistry. Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemistry: Washington, DC, USA, 1990. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: an opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]






| Composition (%) | ||||
|---|---|---|---|---|
| Moisture | Protein | Lipid | Ash | |
| Non-treated skin | 62.22 ± 0.48 | 69.24 ± 0.67 | 2.72 ± 0.18 | 35.13 ± 0.26 |
| Polynomial Equations | R2adj | Topt (°C) | topt (h) | NaOHopt (M) | |
|---|---|---|---|---|---|
| Liquid | Collagen (%) = 87.9 + 26.2 × T + 14.4 × NaOH + 12.7 × t − 5.9 × T × NaOH × t − 7.9 − T2 − 9.6 × NaOH2 − 9.1 × t2 | 0.846 | 25 | 48 | 2 |
| Solid | Collagen (%) = 14.4 − 26.5 × T − 17.8 × NaOH − 14.9 × t + 3.7 × T × NaOH + 7.04 × T × t − 4.5 × NaOH × t + 6.6 × T × NaOH × t + 6.9 × T2 + 11.7 × NaOH2 + 9.3 × t2 | 0.811 | 4 | 2 | 0.1 |
| N° Experiment | T (°C) | Acetic Acid (M) | t (h) | Micromole in Lyophilized Extracted Collagen | ||
|---|---|---|---|---|---|---|
| OHPro | Pro | Gly | ||||
| 1 | 8.26 ((−1)) | 0.36 (−1) | 11.33 (−1) | 65.12 | 102.24 | 367.69 |
| 2 | 20.74 ((1)) | 0.36 (−1) | 11.33 (−1) | 151.34 | 237.70 | 854.61 |
| 3 | 8.26 ((−1)) | 0.84 (1) | 11.33 (−1) | 87.58 | 137.44 | 494.40 |
| 4 | 20.74((1)) | 0.84 (1) | 11.33 (−1) | 144.44 | 226.88 | 815.48 |
| 5 | 8.26 ((−1)) | 0.36 (−1) | 38.67 (1) | 81.01 | 127.18 | 456.98 |
| 6 | 20.74 ((1)) | 0.36 (−1) | 38.67 (1) | 168.79 | 265.12 | 952.75 |
| 7 | 8.26 ((−1)) | 0.84 (1) | 38.67 (1) | 121.52 | 191.05 | 686.78 |
| 8 | 20.74 ((1)) | 0.84 (1) | 38.67 (1) | 174.42 | 274.06 | 985.54 |
| 9 | 4.00 (−1.682) | 0.60 (0) | 25.00 (0) | 68.59 | 107.72 | 387.32 |
| 10 | 25.00 (1.682) | 0.60 (0) | 25.00 (0) | 168.85 | 265.32 | 953.85 |
| 11 | 14.50 (0) | 0.20 (−1.682) | 25.00 (0) | 108.93 | 171.04 | 614.92 |
| 12 | 14.50 (0) | 1.00 (1.682) | 25.00 (0) | 155.82 | 244.70 | 879.74 |
| 13 | 14.50 (0) | 0.60 (0) | 2.00 (−1.682) | 71.32 | 112.12 | 403.03 |
| 14 | 14.50 (0) | 0.60 (0) | 48.00 (1.682) | 131.02 | 205.82 | 740.10 |
| 15 | 14.50 (0) | 0.60 (0) | 25.00 (0) | 116.16 | 182.34 | 655.82 |
| 16 | 14.50 (0) | 0.60 (0) | 25.00 (0) | 131.89 | 207.09 | 744.66 |
| 17 | 14.50 (0) | 0.60 (0) | 25.00 (0) | 139.18 | 218.56 | 785.62 |
| 18 | 14.50 (0) | 0.60 (0) | 25.00 (0) | 158.14 | 248.32 | 892.94 |
| 19 | 14.50 (0) | 0.60 (0) | 25.00 (0) | 141.82 | 221.77 | 797.30 |
| 20 | 14.50 (0) | 0.60 (0) | 25.00 (0) | 134.02 | 210.51 | 756.67 |
| Polynomial Equations | R2adj | Topt (°C) | topt (h) | AcOHopt (M) |
|---|---|---|---|---|
| Pro (µmoles) = 214.4 + 52.1 × T + 16.2 × AcOH + 22.8 × t − 17.1 × t2 | 0.860 | 25 | 34.2 | 1 |
| HPro (µmoles) = 136.5 + 33.1 × T + 10.3 × AcOH + 14.5 × t − 10.9 × t2 | 0.860 | 25 | 34.2 | 1 |
| Gly (µmoles) = 770.7 + 187.1 × T + 58.3 × AcOH + 81.8 × t − 61.4 × t2 | 0.860 | 25 | 34.2 | 1 |
| HPro + Pro (µmoles) = 350.9 + 85.2 × T + 26.5 × AcOH + 37.2 × t − 28.0 × t2 | 0.860 | 25 | 34.2 | 1 |
| Yield (%) = 39.2 + 9.3 × T + 3.1 × AcOH + 4.1 × t − 3.4 × t2 | 0.853 | 25 | 34.2 | 1 |
| Alkaline Pre-Treatment | Acid Extraction | |||||
|---|---|---|---|---|---|---|
| Coded Values | T (°C) | NaOH (M) | t (h) | T (°C) | AcOH (M) | t (h) |
| −1.68 | 4.0 | 0.10 | 2.0 | 4.0 | 0.20 | 2.0 |
| −1 | 8.3 | 0.49 | 11.3 | 8.3 | 0.36 | 11.3 |
| 0 | 14.5 | 1.05 | 25.0 | 14.5 | 0.60 | 25.0 |
| +1 | 20.7 | 1.61 | 38.7 | 20.7 | 0.84 | 38.7 |
| +1.68 | 25.0 | 2.00 | 48.0 | 25.0 | 1.00 | 48.0 |
| Codification: Vc = (Vn − V0)/ΔVn Decodification: Vn = V0 + (ΔVn × Vc) Vn = natural value of the variable to codify ΔVn = increment of Vn for unit of Vc V0 = natural value in the centre of the domain Vc = codified value of the variable | ||||||
| The Model is Acceptable When: | |
|---|---|
| F1 = Model/Total error | F1 ≥ |
| F2 = (Model + Lack of fitting)/Model | F2 ≤ |
| F3 = Total error/Experimental error | F3 ≤ |
| F4 = Lack of fitting/Experimental error | F4 ≤ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; G. Sotelo, C. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology. Mar. Drugs 2019, 17, 40. https://0-doi-org.brum.beds.ac.uk/10.3390/md17010040
Blanco M, Vázquez JA, Pérez-Martín RI, G. Sotelo C. Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology. Marine Drugs. 2019; 17(1):40. https://0-doi-org.brum.beds.ac.uk/10.3390/md17010040
Chicago/Turabian StyleBlanco, María, José Antonio Vázquez, Ricardo I. Pérez-Martín, and Carmen G. Sotelo. 2019. "Collagen Extraction Optimization from the Skin of the Small-Spotted Catshark (S. canicula) by Response Surface Methodology" Marine Drugs 17, no. 1: 40. https://0-doi-org.brum.beds.ac.uk/10.3390/md17010040