Anti-Obesity and Anti-Diabetic Effects of Ishige okamurae
Abstract
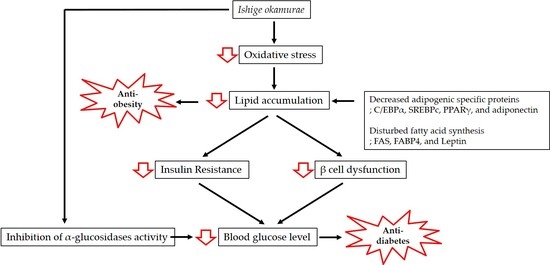
:1. Introduction
2. Anti-Obesity and Anti-Diabetic Properties of IO Extract
3. Composition of IO
3.1. Anti-Obesity Effect of IO
3.2. Anti-Diabetic Activity of IO
4. Potential Nutraceutical Use of IO
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. World Health Organization Obesity and Overweight Fact Sheet. 2016. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 29 March 2019).
- Scully, T. Society at large: The increasing prevalence of obesity is a worldwide phenomenon, affecting peoples from diverse cultural and economic backgrounds. Nature 2014, 508, S50. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Obesity and oxidative stress: A direct link to CVD? Am. Heart Assoc. 2003, 23, 365–367. [Google Scholar] [CrossRef]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pr. 2013, 7, 330–341. [Google Scholar] [CrossRef]
- Maddux, B.A.; See, W.; Lawrence, J.C.; Goldfine, A.L.; Goldfine, I.D.; Evans, J.L. Protection against oxidative stress-induced insulin resistance in rat L6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes 2001, 50, 404–410. [Google Scholar] [CrossRef]
- Nakazono, K.; Watanabe, N.; Matsuno, K.; Sasaki, J.; Sato, T.; Inoue, M. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl. Acad. Sci. USA 1991, 88, 10045–10048. [Google Scholar] [CrossRef] [Green Version]
- Keaney, J.F., Jr.; Larson, M.G.; Vasan, R.S.; Wilson, P.W.; Lipinska, I.; Corey, D.; Massaro, J.M.; Sutherland, P.; Vita, J.A.; Benjamin, E.J. Obesity and systemic oxidative stress: Clinical correlates of oxidative stress in the Framingham Study. Arterioscl. Throm. Vasc. 2003, 23, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef]
- Maritim, A.; Sanders, A.; Watkins Iii, J. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Jain, S.K. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J. Biol. Chem. 1989, 264, 21340–21345. [Google Scholar]
- Jain, S.K.; Levine, S.N.; Duett, J.; Hollier, B. Elevated lipid peroxidation levels in red blood cells of streptozotocin-treated diabetic rats. Metabolism 1990, 39, 971–975. [Google Scholar] [CrossRef]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress–induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef]
- Chang, M.S.; Oh, M.S.; Jung, K.J.; Park, S.; Choi, S.B.; Ko, B.-S.; Park, S.K. Effects of Okchun-San, a herbal formulation, on blood glucose levels and body weight in a model of Type 2 diabetes. J. Ethnopharmacol. 2006, 103, 491–495. [Google Scholar] [CrossRef]
- Heo, S.-J.; Kim, J.-P.; Jung, W.-K.; Lee, N.-H.; Kang, H.-S.; Jun, E.-M.; Park, S.-H.; Kang, S.-M.; Lee, Y.-J.; Park, P.-J. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J. Microbiol. Biotechnol. 2008, 18, 676–681. [Google Scholar]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Lee, K.M.; Boo, G.H.; Riosmena-Rodriguez, R.; Shin, J.A.; Boo, S.M. Classification of the genus Ishige (ishigeales, phaeophyceae) in the north Pacific Ocean with recognition of Ishige foliacea based on plastid rbcl and mitochondrial cox3 gene sequences 1. J. Phycol. 2009, 45, 906–913. [Google Scholar] [CrossRef]
- Ahn, S.-M.; Hong, Y.-K.; Kwon, G.-S.; Sohn, H.-Y. Evaluation of antioxidant and nitrite scavenging activity of seaweed extracts. J. Life Sci. 2011, 21, 576–583. [Google Scholar] [CrossRef]
- Min, K.-H.; Kim, H.-J.; Jeon, Y.-J.; Han, J.-S. Ishige okamurae ameliorates hyperglycemia and insulin resistance in C57BL/KsJ-db/db mice. Diabetes Res. Clin. Pr. 2011, 93, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Kang, N.; Ko, S.-C.; Kim, Y.-B.; Jeon, Y.-J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Casuso, R.A.; Huertas, J.R. Antioxidant Supplements in Obesity and Metabolic Syndrome: Angels or Demons. In Obesity; Elsevier: Amsterdam, The Netherlands, 2018; pp. 263–275. [Google Scholar]
- Acin-Perez, R.; Enriquez, J.A. The function of the respiratory supercomplexes: The plasticity model. BBA-Bioenergetics 2014, 1837, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.-J.; Jeon, Y.-J. Radical scavenging capacity and cytoprotective effect of enzymatic digests of Ishige okamurae. J. Appl. Phycol. 2008, 20, 1087–1095. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS 2017, 13, 851. [Google Scholar] [CrossRef]
- Holguin, F.; Fitzpatrick, A. Obesity, asthma, and oxidative stress. J. Appl. Physiol. 2009, 108, 754–759. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.-W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef] [PubMed]
- Fajas, L. Adipogenesis: A cross-talk between cell proliferation and cell differentiation. Ann. Med. 2003, 35, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-Y.; Cheon, Y.-P. Suppressive Effects of an Ishige okamurae extract on 3T3-L1 Preadipocyte Differentiation. Dev. Reprod. 2013, 17, 451. [Google Scholar] [CrossRef] [PubMed]
- Polvani, S.; Tarocchi, M.; Tempesti, S.; Bencini, L.; Galli, A. Peroxisome proliferator activated receptors at the crossroad of obesity, diabetes, and pancreatic cancer. World J. Gastroenterol. 2016, 22, 2441. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Rosen, E.D.; Sarraf, P.; Troy, A.E.; Bradwin, G.; Moore, K.; Milstone, D.S.; Spiegelman, B.M.; Mortensen, R.M. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–617. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, J.-H.; Kim, E.J.; Yang, H.J.; Park, J.-S.; Hong, S.-K. Anti-obesity and anti-diabetic effect of neoagarooligosaccharides on high-fat diet-induced obesity in mice. Mar. Drugs 2017, 15, 90. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Lee, K.; Song, J.-H.; Chei, S.; Lee, B.-Y. Ishige okamurae extract suppresses obesity and hepatic steatosis in high fat diet-induced obese mice. Nutrients 2018, 10, 1802. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.A.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484. [Google Scholar] [CrossRef]
- Uno, K.; Katagiri, H.; Yamada, T.; Ishigaki, Y.; Ogihara, T.; Imai, J.; Hasegawa, Y.; Gao, J.; Kaneko, K.; Iwasaki, H. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science 2006, 312, 1656–1659. [Google Scholar] [CrossRef]
- Matsuoka, T.-A.; Kajimoto, Y.; Watada, H.; Kaneto, H.; Kishimoto, M.; Umayahara, Y.; Fujitani, Y.; Kamada, T.; Kawamori, R.; Yamasaki, Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J. Clin. Investig. 1997, 99, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Rudich, A.; Tirosh, A.; Potashnik, R.; Hemi, R.; Kanety, H.; Bashan, N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998, 47, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Kodama, H.; Fujita, M.; Yamazaki, M.; Yamaguchi, I. The possible role of age-related increase in the plasma glucagon/insulin ratio in the enhanced hepatic gluco neogenesis and hyperglycemia in genetically diabetic (C57BL/KsJ-db/db) mice. JPN J. Pharmacol. 1994, 66, 281–287. [Google Scholar] [CrossRef]
- Haffner, S.M.; Miettinen, H.; Stern, M.P. The homeostasis model in the San Antonio heart study. Diabetes Care 1997, 20, 1087–1092. [Google Scholar] [CrossRef]
- Tahara, Y.; Shima, K. Kinetics of HbA1c, glycated albumin, and fructosamine and analysis of their weight functions against preceding plasma glucose level. Diabetes Care 1995, 18, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef]
- Handique, J.; Baruah, J. Polyphenolic compounds: An overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 2001–2002: Marine compounds with anthelmintic, antibacterial, anticoagulant, antidiabetic, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2005, 140, 265–286. [Google Scholar] [Green Version]
- Kong, C.-S.; Kim, J.-A.; Yoon, N.-Y.; Kim, S.-K. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Lee, S.-H.; Kim, S.-K. Phlorotannins from Ishige okamurae and their acetyl-and butyrylcholinesterase inhibitory effects. J. Funct. Foods 2009, 1, 331–335. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, H.-S.; Ko, J.-Y.; Kim, C.-Y.; Lee, J.-H.; Jeon, Y.-J. A single-step isolation of useful antioxidant compounds from Ishige okamurae by using centrifugal partition chromatography. Fish Aquat. Sci. 2016, 19, 22. [Google Scholar] [CrossRef]
- Ryu, B.; Jiang, Y.; Kim, H.-S.; Hyun, J.-M.; Lim, S.-B.; Li, Y.; Jeon, Y.-J. Ishophloroglucin A, a novel phlorotannin for standardizing the anti-α-glucosidase activity of Ishige okamurae. Mar. Drugs 2018, 16, 436. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.-J.; Li, Y.; Kim, M.-M.; Lee, S.-H.; Kim, S.-K. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Jeon, Y.-J. Evaluation of diphlorethohydroxycarmalol isolated from Ishige okamurae for radical scavenging activity and its protective effect against H2O2-induced cell damage. Process Biochem. 2009, 44, 412–418. [Google Scholar] [CrossRef]
- Kumar, S.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef]
- Liu, C.-L.; Liang, A.-L.; Hu, M.-L. Protective effects of fucoxanthin against ferric nitrilotriacetate-induced oxidative stress in murine hepatic BNL CL. 2 cells. Toxicol. In Vitro 2011, 25, 1314–1319. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-C.; Lee, S.-H.; Lee, W.-W.; Kang, N.; Kim, E.-A.; Kim, S.Y.; Lee, D.H.; Kim, D.; Jeon, Y.-J. Protective effect of fucoxanthin isolated from Ishige okamurae against high-glucose induced oxidative stress in human umbilical vein endothelial cells and zebrafish model. J. Funct. Foods 2014, 11, 304–312. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.-S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2010, 425, 215–224. [Google Scholar] [CrossRef]
- Choi, H.S.; Jeon, H.J.; Lee, O.H.; Lee, B.Y. Dieckol, a major phlorotannin in Ecklonia cava, suppresses lipid accumulation in the adipocytes of high-fat diet-fed zebrafish and mice: Inhibition of early adipogenesis via cell-cycle arrest and AMPKα activation. Mol. Nutr. Food Res. 2015, 59, 1458–1471. [Google Scholar] [CrossRef]
- Chan, C.Y.; Wei, L.; Castro-Muñozledo, F.; Koo, W.L. (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion by inhibition of cell proliferation and suppression of adipose phenotype expression. Life Sci. 2011, 89, 779–785. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, K.-Y.; Peng, K.-Y.; Day, Y.-J.; Hung, L.-M. Resveratrol exerts anti-obesity effects in high-fat diet obese mice and displays differential dosage effects on cytotoxicity, differentiation, and lipolysis in 3T3-L1 cells. Endocr. J. 2016, 63, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-C.; Ding, Y.; Kim, H.-S.; Jeon, Y.-J.; Lee, S.-H. Inhibition of Adipogenesis by Diphlorethohydroxycarmalol (DPHC) through AMPK activation in adipocytes. Mar. Drugs 2019, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Jeon, Y.J.; Kim, H.J.; Han, J.S. Effect of Diphlorethohydroxycarmalol isolated from ishige okamurae on apoptosis in 3 t3-L1 preadipocytes. Phytother. Res. 2013, 27, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M. Carbohydrate digestion and absorption: Role of the small intestine. N. Engl. J. Med. 1975, 292, 1225–1230. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Li, M.; Zhu, F.; Liu, F.-L.; Huang, J.-B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.-M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Han, J.-S.; Kim, H.-J.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Choi, J.-I.; Heo, S.-J.; Park, M.-H.; Park, P.-J.; Jeon, B.-T.; Kim, S.-K.; Han, J.-S.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Pae (Ishige okamurae) protects high glucose-induced damage in RINm5F pancreatic β cells via its antioxidant effects. Food Sci. Biotechnol. 2012, 21, 239–246. [Google Scholar] [CrossRef]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Concepts and strategy of functional food science: The European perspective. Am. J. Clin. Nutr. 2000, 71, 1660S–1664S. [Google Scholar] [CrossRef]
- Barrow, C.; Shahidi, F. Marine Nutraceuticals and Functional Foods; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Zou, Y.; Li, Y.; Kim, M.-M.; Lee, S.-H.; Kim, S.-K. Ishigoside, a new glyceroglycolipid isolated from the brown alga Ishige okamurae. Biotechnol. Bioprocess Eng. 2009, 14, 20–26. [Google Scholar] [CrossRef]
- Bae, M.J.; Karadeniz, F.; Ahn, B.-N.; Kong, C.-S. Evaluation of effective MMP inhibitors from eight different brown algae in human fibrosarcoma HT1080 cells. PNF 2015, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Rajapakse, N.; Kim, S.K. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-κB transcription factor in RAW 264.7 cells. Phytother. Res. 2009, 23, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kang, N.; Kim, E.-A.; Yang, H.-W.; Oh, J.-Y.; Fernando, I.P.S.; Kim, K.-N.; Ahn, G.; Jeon, Y.-J. Radioprotective effects of a polysaccharide purified from Lactobacillus plantarum-fermented Ishige okamurae against oxidative stress caused by gamma ray-irradiation in zebrafish in vivo model. J. Funct. Foods 2017, 28, 83–89. [Google Scholar] [CrossRef]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Lee, S.-H.; Park, P.-J.; Kang, D.-H.; Oh, C.; Kim, D.-W.; Han, J.-S.; Jeon, Y.-J. Protective effect of diphlorethohydroxycarmalol isolated from Ishige okamurae against high glucose-induced-oxidative stress in human umbilical vein endothelial cells. Food Chem. Toxicol. 2010, 48, 1448–1454. [Google Scholar] [CrossRef]
- Heo, S.-J.; Cha, S.-H.; Kim, K.-N.; Lee, S.-H.; Ahn, G.; Kang, D.-H.; Oh, C.; Choi, Y.-U.; Affan, A.; Kim, D. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H 2 O 2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Ahn, M.J.; Yoon, K.D.; Kim, C.; Kim, J.; Shin, C.G.; Kim, J. Inhibitory activity on HIV-1 reverse transcriptase and integrase of a carmalol derivative from a brown Alga, Ishige okamurae. Phytother. Res. 2006, 20, 711–713. [Google Scholar] [CrossRef]
- Kim, K.-N.; Heo, S.-J.; Yoon, W.-J.; Kang, S.-M.; Ahn, G.; Yi, T.-H.; Jeon, Y.-J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Milatovic, D.; Dettbarn, W.-D. Modification of acetylcholinesterase during adaptation to chronic, subacute paraoxon application in rat. Toxicol. Appl. Pharmacol. 1996, 136, 20–28. [Google Scholar] [CrossRef]
- Schetinger, M.R.; Porto, N.M.; Moretto, M.B.; Morsch, V.M.; da Rocha, J.B.T.; Vieira, V.; Moro, F.; Neis, R.T.; Bittencourt, S.; Bonacorso, H.G. New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem. Res. 2000, 25, 949–955. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Yu, Q.-S.; Zhu, X.; Holloway, H.W.; Perry, T.; Lee, B.; Ingram, D.K.; Lahiri, D.K. A new therapeutic target in Alzheimer’s disease treatment: Attention to butyrylcholinesterase. Curr. Med. Res. Opin. 2001, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M. Fucoxanthin as a bioactive and nutritionally beneficial marine carotenoid. Carotenoid Sci. 2006, 10, 15–28. [Google Scholar]
- Pahl, H.L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene 1999, 18, 6853. [Google Scholar] [CrossRef] [PubMed]
- Uto, T.; Fujii, M.; Hou, D.-X. 6-(Methylsulfinyl) hexyl isothiocyanate suppresses inducible nitric oxide synthase expression through the inhibition of Janus kinase 2-mediated JNK pathway in lipopolysaccharide-activated murine macrophages. Biochem. Pharmacol. 2005, 70, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Coussens, L.M. Cancer: An inflammatory link. Nature 2004, 431, 405. [Google Scholar] [CrossRef] [PubMed]

| EC50 (µM ± SD) | ||||
|---|---|---|---|---|
| DPPH | Hydroxyl | Alkyl | Superoxide | |
| 6,6′-bieckol | 9.1 ± 0.4 | 23.7 ± 1.1 | 17.3 ± 1.0 | 15.4 ± 0.9 |
| Diphlorethohydroxycarmalol (DPHC) | 10.5 ± 0.5 | 27.1 ± 0.9 | 18.8 ± 1.2 | 16.7 ± 0.6 |
| Phloroglucinol | Not determined | 408.5 ± 3.7 | 103.5 ± 1.9 | 124.7 ± 2.4 |
| Functional Ingredient | Bioactivities | References |
|---|---|---|
| Methanolic extract | Antioxidant, anti-MMP, and anti-diabetic | [21,22,74] |
| Ethanolic extract | Anti-inflammatory | [75] |
| Enzymatic extract | Antioxidant | [26] |
| Fermented extract | Radioprotective and antioxidant | [76] |
| 6,6′-Bieckol | Cholinesterase inhibition | [49] |
| Diphlorethohydroxycarmalol (DPHC) | Antioxidant, anti-cancer, anti-HIV, anti-obesity, and anti-diabetic | [52,53,64,68,69,77,78,79] |
| Fucoxanthin | Antioxidant, anti-inflammatory | [58,80] |
| Ishigoside | Antioxidant | [20] |
| Ishophloroglucin A (IPA) | α-glucosidases inhibition | [51] |
| Phloroglucinol | Cholinesterase inhibition | [49] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-W.; Fernando, K.H.N.; Oh, J.-Y.; Li, X.; Jeon, Y.-J.; Ryu, B. Anti-Obesity and Anti-Diabetic Effects of Ishige okamurae. Mar. Drugs 2019, 17, 202. https://0-doi-org.brum.beds.ac.uk/10.3390/md17040202
Yang H-W, Fernando KHN, Oh J-Y, Li X, Jeon Y-J, Ryu B. Anti-Obesity and Anti-Diabetic Effects of Ishige okamurae. Marine Drugs. 2019; 17(4):202. https://0-doi-org.brum.beds.ac.uk/10.3390/md17040202
Chicago/Turabian StyleYang, Hye-Won, K.H.N. Fernando, Jae-Young Oh, Xining Li, You-Jin Jeon, and BoMi Ryu. 2019. "Anti-Obesity and Anti-Diabetic Effects of Ishige okamurae" Marine Drugs 17, no. 4: 202. https://0-doi-org.brum.beds.ac.uk/10.3390/md17040202