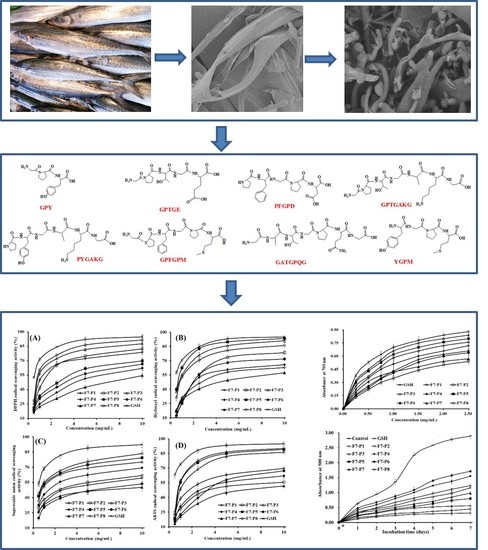
Eight Collagen Peptides from Hydrolysate Fraction of Spanish Mackerel Skins: Isolation, Identification, and In Vitro Antioxidant Activity Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of APs from Collagen Hydrolysate Fraction of F7
2.1.1. Gel Filtration Chromatography of F7 Using Superdex® Peptide 10/300 GL Column
2.1.2. Isolation of APs from FSP-III by RP-HPLC
2.2. Determination of AA Sequence
2.3. Antioxidant Activity
2.3.1. Radical Scavenging Activity
DPPH• Scavenging Activity
HO• Scavenging Activity
• Scavenging Activity
ABTS+• Scavenging Activity
2.3.2. Reducing Power
2.3.3. Lipid Peroxidation Inhibition Assay
2.3.4. Discussion on Structure-Activity Relationship of APs
3. Experimental Section
3.1. Materials
3.2. Isolation of APs from Hydrolysate Fraction of F7
3.3. Analysis of Antioxidant Activity
3.3.1. Radical Scavenging Activity
DPPH• Scavenging Activity
HO• Scavenging Activity
• Scavenging Activity
ABTS+• Scavenging Activity
3.3.2. Reducing Power Assay
3.3.3. Lipid Peroxidation Inhibition Assay
3.4. Determination of Molecular Mass and AA Sequences
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, W.H.; Luo, Q.B.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy). J. Funct. Foods 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Jin, J.E.; Ahn, C.B.; Je, J.Y. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed ark shell (Scapharca subcrenata). Process Biochem. 2018, 72, 170–176. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.Q.; Hu, F.Y.; Wang, B. Preparation and identification of antioxidant peptides from protein hydrolysate of skate (Raja porosa) cartilage. J. Funct. Foods 2016, 25, 220–230. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Chi, C.; Hu, F.; Deng, S.; Ma, J. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Chi, C.F.; Ma, J.H.; Luo, H.Y.; Xu, Y.F. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chem. 2013, 138, 1713–1719. [Google Scholar] [CrossRef]
- Gimenez, B.; Aleman, A.; Montero, P.; Gomez-Guillen, M.C. Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chem. 2009, 114, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Jridi, M.; Bardaa, S.; Moalla, D.; Rebaii, T.; Souissi, N.; Sahnoun, Z.; Nasri, M. Microstructure, rheological and wound healing properties of collagen-based gel from cuttlefish skin. Int. J. Biol. Macromol. 2015, 77, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Zhang, B.; Deng, S.G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Ren, X.J.; Deng, S.G.; Wu, C.W. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem. 2015, 168, 662–667. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H. Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth Factor-β/Smad signaling pathway. J. Photochem. Photobiol. B 2016, 162, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Zhang, L.; Tao, J.; Chi, C.F.; Wang, B. Isolation, identification, and antihypertensive activity evaluation of eight peptides from the protein hydrolysate of Antarctic krill (Euphausia superba). Food Res. Int. 2019, 121, 197–204. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Zeng, L.; Yang, Z.S.; Huang, F.F.; Ding, G.F.; Wang, B. Anti-fatigue effect by peptide fraction from protein hydrolysate of croceine croaker (Pseudosciaena crocea) swim bladder through inhibiting the oxidative reactions including DNA damage. Mar. Drugs 2016, 14, 221. [Google Scholar] [CrossRef]
- Li, Z.R.; Wang, B.; Chi, C.F.; Zhang, Q.H.; Gong, Y.D.; Tang, J.J.; Luo, H.Y.; Ding, G.F. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius). Food Hydrocoll. 2013, 31, 103–113. [Google Scholar] [CrossRef]
- Chi, C.F.; Cao, Z.H.; Wang, B.; Hu, F.Y.; Li, Z.R.; Zhang, B. Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.R.; Chi, C.F.; Zhang, Q.H.; Luo, H.Y. Preparation and evaluation of antioxidant peptides from ethanol-soluble proteins hydrolysate of Sphyrna lewini muscle. Peptides 2012, 36, 240–250. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.S.; Ahn, C.B.; Je, J.Y. Partial purification and identification of three antioxidant peptides with hepatoprotective effects from blue mussel (Mytilus edulis) hydrolysate by peptic hydrolysis. J. Funct. Foods 2016, 20, 88–95. [Google Scholar] [CrossRef]
- Ahn, C.B.; Cho, Y.S.; Je, J.Y. Purification and anti-inflammatory action of tripeptide from salmon pectoral fin byproduct protein hydrolysate. Food Chem. 2015, 168, 151–156. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionizationmass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Ahn, C.B.; Kim, J.G.; Je, J.Y. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014, 147, 78–83. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Martinović, N.; Abramovič, H.; Ulrih, N.P. Inhibition of copper-induced lipid peroxidation by sinapic acid and its derivatives in correlation to their effect on the membrane structural properties. BBA 2019, 1861, 1–8. [Google Scholar] [CrossRef]
- Osawa, T.; Namiki, M. Natural antioxidant isolated from eucalyptus leaf waxes. J. Agric. Food Chem. 1985, 33, 770–780. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, Y.; Li, L. Relationship between primary structure or spatial conformation and functional activity of antioxidant peptides from Pinctada fucata. Food Chem. 2018, 264, 108–117. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2018. [Google Scholar] [CrossRef]
- Feng, Y.X.; Ruan, G.R.; Jin, F.; Xu, J.; Wang, F.J. Purification, identification, and synthesis of five novel antioxidant peptides from Chinese chestnut (Castanea mollissima Blume) protein hydrolysates. LWT Food Sci. Technol. 2018, 92, 40–46. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Asoodeh, A.; Chamani, J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods 2012, 4, 278–286. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Xu, H.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemosa. J. Appl. Phycol. 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- He, Y.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Ten new pentapeptides from protein hydrolysate of miiuy croaker (Miichthys miiuy) muscle: Preparation, identification, and antioxidant activity evaluation. LWT Food Sci. Technol. 2019, 105, 1–8. [Google Scholar] [CrossRef]
- Li, X.R.; Chi, C.F.; Li, L.; Wang, B. Purification and identification of antioxidant peptides from protein hydrolysate of scalloped hammerhead (Sphyrna lewini) cartilage. Mar. Drugs 2017, 15, 61. [Google Scholar] [CrossRef]





| RT (min) | AA Sequence | Theoretical Mass/Observed Mass (Da) | |
|---|---|---|---|
| F7-P1 | 4.951 | GPY | 335.36/335.31 |
| F7-P2 | 8.561 | GPTGE | 459.45/459.47 |
| F7-P3 | 9.704 | PFGPD | 531.56/531.52 |
| F7-P4 | 10.245 | GPTGAKG | 586.64/586.65 |
| F7-P5 | 10.804 | PYGAKG | 591.66/591.69 |
| F7-P6 | 11.892 | GATGPQG | 586.60/586.61 |
| F7-P7 | 13.389 | GPFGPM | 604.72/604.73 |
| F7-P8 | 13.612 | YGPM | 466.55/466.50 |
| EC50 (mg/mL) | ||||
|---|---|---|---|---|
| DPPH• | HO• | • | ABTS+• | |
| F7-P1 | 1.01 ± 0.09 a | 3.22 ± 0.21 a | 3.98 ± 0.26 a | 2.12 ± 0.16 a |
| F7-P2 | 1.46 ± 0.12 b | 2.13 ± 0.15 b | 2.96 ± 0.19 b | 4.51 ± 0.24 b |
| F7-P3 | 0.80 ± 0.09 a | 0.81 ± 0.05 c | 0.91 ± 0.08 c | 0.86 ± 0.05 c |
| F7-P4 | 4.63 ± 0.21 c | 2.27 ± 0.14 b | 1.73 ± 0.11 d | 3.27 ± 0.27 d |
| F7-P5 | 3.02 ± 0.19 d | 0.66 ± 0.08 c | 0.80 ± 0.06 c | 1.07 ± 0.10 c |
| F7-P6 | 3.65 ± 0.30 e | 2.11 ± 0.16 b | 7.21 ± 0.27 e | 3.86 ± 0.19 e |
| F7-P7 | 7.93 ± 0.52 f | 5.24 ± 0.32 d | 9.83 ± 0.35 f | 8.54 ± 0.43 f |
| F7-P8 | 0.72 ± 0.06 a | 0.88 ± 0.09 c | 0.73 ± 0.06 c | 0.82 ± 0.04 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-B.; Zhao, Y.-Q.; Wang, Y.-M.; Chi, C.-F.; Wang, B. Eight Collagen Peptides from Hydrolysate Fraction of Spanish Mackerel Skins: Isolation, Identification, and In Vitro Antioxidant Activity Evaluation. Mar. Drugs 2019, 17, 224. https://0-doi-org.brum.beds.ac.uk/10.3390/md17040224
Zhang J-B, Zhao Y-Q, Wang Y-M, Chi C-F, Wang B. Eight Collagen Peptides from Hydrolysate Fraction of Spanish Mackerel Skins: Isolation, Identification, and In Vitro Antioxidant Activity Evaluation. Marine Drugs. 2019; 17(4):224. https://0-doi-org.brum.beds.ac.uk/10.3390/md17040224
Chicago/Turabian StyleZhang, Jing-Bo, Yu-Qin Zhao, Yu-Mei Wang, Chang-Feng Chi, and Bin Wang. 2019. "Eight Collagen Peptides from Hydrolysate Fraction of Spanish Mackerel Skins: Isolation, Identification, and In Vitro Antioxidant Activity Evaluation" Marine Drugs 17, no. 4: 224. https://0-doi-org.brum.beds.ac.uk/10.3390/md17040224