Structures and Absolute Configurations of Diketopiperazine Alkaloids Chrysopiperazines A–C from the Gorgonian-Derived Penicillium chrysogenum Fungus
Abstract
:1. Introduction
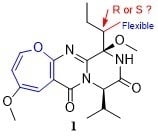
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.3.1. Chrysopiperazine A (1)
3.3.2. Chrysopiperazine B (2)
3.3.3. Chrysopiperazine C (5)
3.4. Preparation and Analysis of Marfey’s Derivatives
3.5. Bioactivity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martins, M.B.; Carvalho, I. Diketopiperazines: Biological activity and synthesis. Tetrahedron 2007, 63, 9923–9932. [Google Scholar] [CrossRef]
- Zhang, P.; Mandi, A.; Li, X.M.; Du, F.Y.; Wang, J.N.; Li, X.; Kurtán, T.; Wang, B.G. Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii. Org. Lett. 2014, 16, 4834–4837. [Google Scholar] [CrossRef]
- Wang, J.F.; He, W.J.; Huang, X.L.; Tian, X.P.; Liao, S.R.; Yang, B.; Wang, F.Z.; Zhou, X.J.; Liu, Y.H. Antifungal new oxepine-containing alkaloids and xanthones from the deep-sea-derived fungus Aspergillus versicolor SCSIO 05879. J. Agric. Food Chem. 2016, 64, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.M.; Wang, J.N.; Wang, B.G. Oxepine-containing diketopiperazine alkaloids from the algal-derived endophytic fungus Paecilomyces variotii EN-291. Helv. Chim. Acta 2015, 98, 800–804. [Google Scholar] [CrossRef]
- Sprogøe, K.; Manniche, S.; Larsen, T.O.; Christophersen, C. Janoxepin and brevicompanine B: Antiplasmodial metabolites from the fungus Aspergillus janus. Tetrahedron 2005, 61, 8718–8721. [Google Scholar] [CrossRef]
- Hou, X.M.; Liang, T.M.; Guo, Z.Y.; Wang, C.Y.; Shao, C.L. Discovery, absolute assignments, and total synthesis of asperversiamides A–C and their potent activity against Mycobacterium marinum. Chem. Commun. 2019, 55, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Li, D.; Shao, C.L.; Deng, D.S.; Wang, C.Y. (±)-Pestalachloride D, an antibacterial racemate of chlorinated benzophenone derivative from a soft coral-derived fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef]
- Jia, Y.L.; Wei, M.Y.; Chen, H.Y.; Guan, F.F.; Wang, C.Y.; Shao, C.L. (+)-and (−)-Pestaloxazine A, a pair of antiviral enantiomeric alkaloid dimers with a symmetric spiro [oxazinane-piperazinedione] skeleton from Pestalotiopsis sp. Org. Lett. 2015, 17, 4216–4219. [Google Scholar] [CrossRef]
- Pan, C.Q.; Shi, Y.T.; Chen, X.G.; Chen, C.A.; Tao, X.Y.; Wu, B. New compounds from a hydrothermal vent crab-associated fungus Aspergillus versicolor XZ-4. Org. Biomol. Chem. 2017, 15, 1155–1163. [Google Scholar] [CrossRef]
- Marfey, P. Determination of D-amino acids. II. Use of a bifunctional reagent, 1, 5-difluoro-2, 4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef]
- Zhu, A.; Yang, M.Y.; Zhang, Y.H.; Shao, C.L.; Wang, C.Y.; Hu, L.D.; Cao, F.; Zhu, H.J. Absolute configurations of 14, 15-hydroxylated prenylxanthones from a marine-derived Aspergillus sp. fungus by chiroptical methods. Sci. Rep.-UK. 2018, 8, 10621. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Meng, Z.H.; Mu, X.; Yue, Y.F.; Zhu, H.J. Absolute configuration of bioactive azaphilones from the marine-derived fungus Pleosporales sp. CF09-1. J. Nat. Prod. 2019, 82, 386–392. [Google Scholar] [CrossRef]
- Cutler, H.G.; Springer, J.P.; Arrendale, R.F.; Arison, B.H.; Cole, P.D.; Roberts, R.G. Cinereain: A novel metabolite with plant growth regulating properties from Botrytis cinerea. Agric. Boil. Chem. 1988, 52, 1725–1733. [Google Scholar] [CrossRef]
- Belofsky, G.N.; Anguera, M.; Jensen, P.R.; Fenical, J.W.; Köck, M. Oxepinamides A–C and fumiquinazolines H–I: Bioactive metabolites from a marine isolate of a fungus of the genus Acremonium. Chem. Eur. J. 2000, 6, 1355–1360. [Google Scholar] [CrossRef]
- Lu, X.H.; Shi, Q.W.; Zheng, Z.H.; Ke, A.B.; Zhang, H.; Huo, C.H.; Ma, Y.; Ren, X.; Li, Y.Y.; Lin, J.; et al. Oxepinamides: Novel liver X receptor agonists from Aspergillus puniceus. Eur. J. Org. Chem. 2011, 2011, 802–807. [Google Scholar] [CrossRef]
- Li, G.Y.; Yang, T.; Luo, Y.G.; Chen, X.Z.; Fang, D.M.; Zhang, G.L. Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org. Lett. 2009, 11, 3714–3717. [Google Scholar] [CrossRef]
- Li, G.Y.; Li, L.M.; Yang, T.; Chen, X.Z.; Fang, D.M.; Zhang, G.L. Four new alkaloids, brevianamides O–R, from the fungus Aspergillus versicolor. Helv. Chim. Acta 2010, 93, 2075–2080. [Google Scholar] [CrossRef]
- Lee, S.U.; Asami, Y.; Lee, D.; Jang, J.; Ahn, J.S.; Oh, H. Protuboxepins A and B and protubonines A and B from the marine-derived fungus Aspergillus sp. SF-5044. J. Nat. Prod. 2011, 74, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Q.; Lin, S.N.; Zhou, H.; Lin, S.T.; Wang, S.Y.; Liu, Y.H. Protuboxepin C and protuboxepin D from the sponge-derived fungus Aspergillus sp. SCSIO XWS02F40. Nat. Prod. Res. 2018, 32, 2510–2515. [Google Scholar] [CrossRef]
- Luo, X.; Chen, C.; Tao, H.; Lin, X.; Yang, B.; Zhou, X.; Liu, Y. Structurally diverse diketopiperazine alkaloids from the marine-derived fungus Aspergillus versicolor SCSIO 41016. Org. Chem. Front. 2019, 6, 736–740. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Afiyatullov, S.S.; Yurchenko, E.A.; Denisenko, V.A.; Kirichuk, N.N.; Dmitrenok, P.S. New metabolites from the algal associated marine-derived fungus Aspergillus carneus. Nat. Prod. Commun. 2013, 8, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure−activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]




| Position | 1 | 2 | 5 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 169.2 | 166.8 | 169.9 | |||
| 2 | 7.04, s | 6.10, s | 6.61, s | |||
| 3 | 88.1 | 90.4 | 88.5 | |||
| 4 | 149.8 | 151.2 | 144.6 | |||
| 6 | 158.3 | 159.5 | 140.4 | |||
| 7 | 129.4 | 7.66, d (9.0) | ||||
| 8 | 144.4 | 6.20, d (6.0) | 144.8 | 6.22, d (6.0) | 125.0 | 7.38, dd (9.0, 2.9) |
| 9 | 115.8 | 5.53, dd (6.0, 1.4) | 115.4 | 5.52, dd (6.0, 1.4) | 159.1 | |
| 10 | 157.3 | 157.4 | 106.2 | 7.65, d (2.9) | ||
| 11 | 94.8 | 5.80, d (1.4) | 94.4 | 5.78, d (1.4) | 121.3 | |
| 12 | 110.5 | 110.6 | 161.3 | |||
| 13 | 161.8 | 161.3 | ||||
| 60.7 | 5.24, d (8.7) | |||||
| 15 | 61.0 | 5.07, d (7.8) | 61.2 | 5.03, d (4.1) | 33.4 | 2.53, m |
| 16 | 33.3 | 2.45, m | 33.1 | 2.38, m | 19.6 | 0.95, d (6.8) |
| 17 | 19.5 | 1.15, d (7.0) | 20.3 | 1.23, d (7.0) | 20.0 | 1.22, d (6.7) |
| 18 | 19.7 | 1.00, d (6.9) | 17.8 | 0.97, d (6.9) | 36.2 | 2.96, m |
| 19 | 36.4 | 2.73, m | 42.1 | 2.50, m | 10.9 | 1.07, d (7.0) |
| 20 | 12.1 | 0.92, d (7.2) | 14.9 | 0.95, d (6.9) | 25.1 | 1.26, m, 1.00, m |
| 21 | 24.8 | 1.11, m | 21.4 | 1.03, m; 1.96, m | 12.4 | 0.93, t (7.0) |
| 22 | 10.6 | 1.04, t (7.0) | 11.9 | 1.00, t (6.9) | 55.8 | 3.96, s |
| 23 | 55.2 | 3.72, s | 55.2 | 3.73, s | 50.9 | 3.27, s |
| 24 | 51.2 | 3.26, s | 50.5 | 2.96, s | ||
| Compd. | Source | Absolute Configuration of C-19 | Method |
|---|---|---|---|
| Oxepinamide A14 | Acremonium sp. | not determined | - |
| Oxepinamide B14 | Acremonium sp. | not determined | - |
| Brevianamide L16 | Aspergillus versicolor | not determined | - |
| Brevianamide O17 | A. versicolor | not determined | - |
| Brevianamide P17 | A. versicolor | not determined | - |
| Protuboxepin A18 | Aspergillus sp. | not determined | - |
| Oxepinamide E15 | A. puniceus | S | X-ray |
| Oxepinamide F15 | A. puniceus | S | biosynthetic origins |
| Versicoloid A3 | A. versicolor | not determined | - |
| Versicoloid B3 | A. versicolor | not determined | - |
| Versicomide D9 | A. versicolor | S | acid hydrolyzed |
| Protuboxepin C19 | Aspergillus sp. | S | X-ray |
| Protuboxepin D19 | Aspergillus sp. | S | biosynthetic origins |
| Chrysopiperazine A | P. chrysogenum | S | VCD |
| Chrysopiperazine B | P. chrysogenum | S | VCD |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.-F.; Mao, N.; Xue, X.-J.; Qi, Y.-X.; Wei, M.-Y.; Wang, C.-Y.; Shao, C.-L. Structures and Absolute Configurations of Diketopiperazine Alkaloids Chrysopiperazines A–C from the Gorgonian-Derived Penicillium chrysogenum Fungus. Mar. Drugs 2019, 17, 250. https://0-doi-org.brum.beds.ac.uk/10.3390/md17050250
Xu W-F, Mao N, Xue X-J, Qi Y-X, Wei M-Y, Wang C-Y, Shao C-L. Structures and Absolute Configurations of Diketopiperazine Alkaloids Chrysopiperazines A–C from the Gorgonian-Derived Penicillium chrysogenum Fungus. Marine Drugs. 2019; 17(5):250. https://0-doi-org.brum.beds.ac.uk/10.3390/md17050250
Chicago/Turabian StyleXu, Wei-Feng, Ning Mao, Xiao-Jia Xue, Yue-Xuan Qi, Mei-Yan Wei, Chang-Yun Wang, and Chang-Lun Shao. 2019. "Structures and Absolute Configurations of Diketopiperazine Alkaloids Chrysopiperazines A–C from the Gorgonian-Derived Penicillium chrysogenum Fungus" Marine Drugs 17, no. 5: 250. https://0-doi-org.brum.beds.ac.uk/10.3390/md17050250