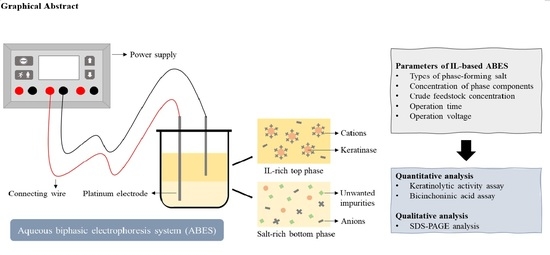
Evaluation of Aqueous Biphasic Electrophoresis System Based on Halide-Free Ionic Liquids for Direct Recovery of Keratinase
Abstract
:1. Introduction
2. Results and Discussion
2.1. Binodal Curve of [EMIM][ESO4]/Salt Biphasic System
2.2. Selection of Types of Salt for Keratinase Recovery
2.3. Selection of Concentration of Phase Components for Keratinase Recovery
2.4. Selection of the Amount of Crude Feedstock Load for Keratinase Recovery
2.5. Selection of Operation Time for Keratinase Recovery
2.6. Selection of Operation Voltage for Keratinase Recovery
2.7. Determination of Purity of Keratinase Recovered from the Halide-Free IL-Based ABES
3. Materials and Methods
3.1. Materials
3.2. Microbial Fermentation of Kytococcus sedentarius TWHKC01 for Keratinase Production
3.3. Construction of Binodal Curve
3.4. Partition Experiment
3.5. Keratinolytic Activity Assay and Bicinchoninic Acid Assay
3.6. Determination of Recovery Yield, Partition Coefficient, Selectivity and Purification Fold
3.7. Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Y.-Y.; Kee, P.E.; Ng, H.-S.; Lan, J.C.-W. Recovery efficiency of a hydrophilic ionic-liquid aqueous biphasic system for the primary purification of cytochrome c from simulated Saccharomyces cerevisiae fermentation broth. Process Biochem. 2020, 94, 110–115. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Hou, Y. Efficient purification of R-phycoerythrin from marine algae (Porphyra yezoensis) based on a deep eutectic solvents aqueous two-phase system. Mar. Drugs 2020, 18, 618. [Google Scholar] [CrossRef]
- Naushad, M.; Alothman, Z.; Khan, D.A.; Ali, M. Effect of ionic liquids on activity, stability and structure of enzymes: A review. Int. J. Biol. Macromol. 2012, 51, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Capela, E.V.; Valente, A.I.; Nunes, J.C.F.; Magalhães, F.F.; Rodríguez, O.; Soto, A.; Freire, M.G.; Tavares, A.P.M. Insights on the laccase extraction and activity in ionic-liquid-based aqueous biphasic systems. Sep. Purif. Technol. 2020, 248, 117052. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Xingang, L. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef] [Green Version]
- McQueen, L.; Lai, D. Ionic liquid aqueous two-phase systems from a pharmaceutical perspective. Front. Chem. 2019, 7, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.-X.; Tang, L.; Fu, T.; Shi, Y.-Q.; Wang, X.; Wang, Y.-Z. Novel phosphorus-containing halogen-free ionic liquids: Effect of sulfonate anion size on its physical properties, biocompatibility, and flame retardancy. RSC Adv. 2016, 6, 52485–52494. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Pei, Y.; Wang, J.; He, M. Design of environmentally friendly ionic liquid aqueous two-phase systems for the efficient and high activity extraction of proteins. Green Chem. 2012, 14, 2941–2950. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Su, S.-G.; Gung, S.-T.; Lin, M.-W.; Lin, Y.-C.; Lai, C.-A.; Sun, I.W. Ionic liquids containing an alkyl sulfate group as potential electrolytes. Electrochim. Acta 2010, 55, 4475–4482. [Google Scholar] [CrossRef]
- Deive, F.J.; Rodríguez, A.; Marrucho, I.M.; Rebelo, L.P.N. Aqueous biphasic systems involving alkylsulfate-based ionic liquids. J. Chem. Thermodyn. 2011, 43, 1565–1572. [Google Scholar] [CrossRef]
- Deive, F.J.; Rivas, M.A.; Rodríguez, A. Sodium carbonate as phase promoter in aqueous solutions of imidazolium and pyridinium ionic liquids. J. Chem. Thermodyn. 2011, 43, 1153–1158. [Google Scholar] [CrossRef]
- Kee, P.E.; Lan, J.C.; Yim, H.S.; Tan, J.S.; Chow, Y.H.; Ng, H.S. Efficiency of ionic liquids-based aqueous two-phase electrophoresis for partition of cytochrome c. Appl. Biochem. Biotechnol. 2020, 191, 376–386. [Google Scholar] [CrossRef]
- Buszewski, B.; Studzińska, S. A review of ionic liquids in chromatographic and electromigration techniques. Chromatographia 2008, 68, 1–10. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Ionic liquids as green solvents and electrolytes for robust chemical sensor development. Acc. Chem. Res. 2012, 45, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Kee, P.E.; Lan, J.C.-W.; Yim, H.S.; Chow, Y.H.; Chen, P.-T.; Ng, H.-S. Efficiency of polymer/salt aqueous two-phase electrophoresis system for recovery of extracellular Kytococcus sedentarius TWHKC01 keratinase. Process Biochem. 2020, 100, 199–206. [Google Scholar] [CrossRef]
- Vidmar, B.; Vodovnik, M. Microbial keratinases: Enzymes with promising biotechnological applications. Food Technol. Biotechnol. 2018, 56, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Nnolim, N.E.; Udenigwe, C.C.; Okoh, A.I.; Nwodo, U.U. Microbial keratinase: Next generation green catalyst and prospective applications. Front. Microbiol. 2020, 11, 580164. [Google Scholar] [CrossRef]
- Sharma, R.; Devi, S. Versatility and commercial status of microbial keratinases: A review. Rev. Environ. Sci. Biotechnol. 2017, 17, 19–45. [Google Scholar] [CrossRef]
- Kerouaz, B.; Jaouadi, B.; Brans, A.; Saoudi, B.; Habbeche, A.; Haberra, S.; Belghith, H.; Gargroui, A.; Ladjama, A. Purification and biochemical characterization of two novel extracellular keratinases with feather-degradation and hide-dehairing potential. Process Biochem. 2021, 106, 137–148. [Google Scholar] [CrossRef]
- Hamiche, S.; Mechri, S.; Khelouia, L.; Annane, R.; El Hattab, M.; Badis, A.; Jaouadi, B. Purification and biochemical characterization of two keratinases from Bacillus amyloliquefaciens S13 isolated from marine brown alga Zonaria tournefortii with potential keratin-biodegradation and hide-unhairing activities. Int. J. Biol. Macromol. 2019, 122, 758–769. [Google Scholar] [CrossRef]
- Kee, P.E.; Yim, H.S.; Kondo, A.; Wong, S.Y.W.; Chen, P.-T.; Lan, J.C.-W.; Ng, H.S. Incorporation of electric fields to ionic liquids-based aqueous biphasic system for enhanced recovery of extracellular Kytococcus sedentarius TWHKC01 keratinase. J. Taiwan Inst. Chem. Eng. 2021, in press. [Google Scholar] [CrossRef]
- Ng, H.S.; Ooi, C.W.; Show, P.L.; Tan, C.P.; Ariff, A.; Moktar, M.N.; Ng, E.-P.; Ling, T.C. Recovery of Bacillus cereus cyclodextrin glycosyltransferase using ionic liquid-based aqueous two-phase system. Sep. Purif. Technol. 2014, 138, 28–33. [Google Scholar] [CrossRef]
- Wan, P.K.; Lan, J.C.-W.; Chen, P.-W.; Tan, J.S.; Ng, H.S. Recovery of intracellular ectoine from Halomonas salina cells with poly(propylene) glycol/salt aqueous biphasic system. J. Taiwan Inst. Chem. Eng. 2018, 82, 28–32. [Google Scholar] [CrossRef]
- Li, Z.; Pei, Y.; Wang, H.; Fan, J.; Wang, J. Ionic liquid-based aqueous two-phase systems and their applications in green separation processes. Trends Anal. Chem. 2010, 29, 1336–1346. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, J.; Wu, K.; Xuan, X.; Lu, X. Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep. Purif. Technol. 2009, 64, 288–295. [Google Scholar] [CrossRef]
- Dreyer, S.; Salim, P.; Kragl, U. Driving forces of protein partitioning in an ionic liquid-based aqueous two-phase system. Biochem. Eng. J. 2009, 46, 176–185. [Google Scholar] [CrossRef]
- Ng, H.S.; Wan, P.K.; Ng, T.-C.; Lan, J.C.-W. Primary purification of intracellular Halomonas salina ectoine using ionic liquids-based aqueous biphasic system. J. Biosci. Bioeng. 2020, 130, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-Q.; Li, Z.; Lü, Y.-H.; Yang, Z. Specific ion effects of ionic liquids on enzyme activity and stability. Green Chem. 2011, 13, 1860–1868. [Google Scholar] [CrossRef]
- Gogoi, G.; Hazarika, S. Ionic liquid-mediated aqueous two-phase system to enhance the partitioning of lignin. Can. J. Chem. Eng. 2019, 97, 2527–2535. [Google Scholar] [CrossRef]
- Zhai, S.L.; Luo, G.S.; Liu, J.G. Aqueous two-phase electrophoresis for separation of amino acids. Sep. Purif. Technol. 2001, 21, 197–203. [Google Scholar] [CrossRef]
- Zhai, S.; Luo, G.; Liu, J. Selective recovery of amino acids by aqueous two-phase electrophoresis. Chem. Eng. J. 2001, 83, 55–59. [Google Scholar] [CrossRef]
- Münchow, G.; Hardt, S.; Kutter, J.; Drese, K. Protein transport and concentration by electrophoresis in two-phase microflows. J. Assoc. Lab. Autom. 2006, 11, 368–373. [Google Scholar] [CrossRef]
- Münchow, G.; Hardt, S.; Kutter, J.; Drese, K. Electrophoretic partitioning of proteins in two-phase microflows. Lab Chip 2007, 7, 98–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, D.K.; Das, A.; Thatoi, H.; Mondal, K.C.; Mohapatra, P.K. Keratinase production and biodegradation of whole chicken feather keratin by a newly isolated bacterium under submerged fermentation. Appl. Biochem. Biotechnol. 2012, 167, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.; Lan, J.; Tan, J.S.; Yim, H.S.; Ng, H.S. Aqueous biphasic system for the partial purification of Bacillus subtilis carboxymethyl cellulase. Process Biochem. 2017, 58, 276–281. [Google Scholar] [CrossRef]
 ) and potassium phosphate (
) and potassium phosphate (  ).
).
 ) and potassium phosphate (
) and potassium phosphate (  ).
).




| Phase Component | Ke | YT, % | PFT | |
|---|---|---|---|---|
| IL | Salt | |||
| [EMIM][ESO4] | Carbonate | 3.72 ± 0.24 | 80.60 ± 1.05 | 1.21 ± 0.03 |
| [EMIM][ESO4] | Phosphate | 1.37 ± 0.10 | 73.06 ± 1.34 | 0.91 ± 0.03 |
| Concentration, % (w/w) | Ke | YT, % | PFT | |
|---|---|---|---|---|
| [EMIM][ESO4] | Carbonate | |||
| 15.0 | 17.5 | 4.94 ± 0.26 | 75.77 ± 0.99 | 2.07 ± 0.04 |
| 20.0 | 5.29 ± 0.56 | 78.80 ± 1.50 | 2.38 ± 0.06 | |
| 22.5 | 2.63 ± 0.11 | 59.71 ± 0.99 | 2.30 ± 0.09 | |
| 25.0 | 2.02 ± 0.14 | 53.17 ± 1.70 | 1.98 ± 0.07 | |
| 20.0 | 15.0 | 3.29 ± 0.31 | 74.38 ± 1.90 | 1.45 ± 0.11 |
| 17.5 | 4.48 ± 0.18 | 78.12 ± 0.68 | 1.47 ± 0.05 | |
| 20.0 | 2.11 ± 0.06 | 65.32 ± 0.61 | 1.13 ± 0.05 | |
| 22.5 | 1.44 ± 0.04 | 56.09 ± 0.75 | 1.00 ± 0.05 | |
| 25.0 | 10.0 | 1.78 ± 0.10 | 75.81 ± 1.00 | 1.28 ± 0.03 |
| 12.5 | 2.87 ± 0.20 | 76.12 ± 1.24 | 1.57 ± 0.06 | |
| 15.0 | 4.44 ± 0.23 | 81.54 ± 0.76 | 1.46 ± 0.05 | |
| 17.5 | 3.31 ± 0.33 | 74.45 ± 1.93 | 1.41 ± 0.10 | |
| 20.0 | 1.93 ± 0.23 | 62.85 ± 2.91 | 1.20 ± 0.06 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kee, P.E.; Yim, H.S.; Kondo, A.; Lan, J.C.-W.; Ng, H.S. Evaluation of Aqueous Biphasic Electrophoresis System Based on Halide-Free Ionic Liquids for Direct Recovery of Keratinase. Mar. Drugs 2021, 19, 463. https://0-doi-org.brum.beds.ac.uk/10.3390/md19080463
Kee PE, Yim HS, Kondo A, Lan JC-W, Ng HS. Evaluation of Aqueous Biphasic Electrophoresis System Based on Halide-Free Ionic Liquids for Direct Recovery of Keratinase. Marine Drugs. 2021; 19(8):463. https://0-doi-org.brum.beds.ac.uk/10.3390/md19080463
Chicago/Turabian StyleKee, Phei Er, Hip Seng Yim, Akihiko Kondo, John Chi-Wei Lan, and Hui Suan Ng. 2021. "Evaluation of Aqueous Biphasic Electrophoresis System Based on Halide-Free Ionic Liquids for Direct Recovery of Keratinase" Marine Drugs 19, no. 8: 463. https://0-doi-org.brum.beds.ac.uk/10.3390/md19080463