Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanofiller Preparation
2.1.1. Graphene Oxide
2.1.2. Porous Graphene Oxide
2.1.3. Graphene Oxide Functionalized with Polyetheramine
2.2. Membranes Preparation: “Double-Solvent Compatibilization”
2.3. Materials Characterization
2.3.1. XPS
2.3.2. SEM Analysis
2.3.3. DSC
- (1)
- Cooling from room temperature to −80 °C
- (2)
- Heating from −80 to 250 °C
- (3)
- Cooling from 250 to −80 °C
- (4)
- Heating from −80 to 250 °C
2.3.4. Permeation Test
3. Results and Discussion
3.1. XPS
3.2. SEM
3.3. DSC
3.4. Permeation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC—Intergovernmental Panel on Climate Change Global Warming Potential Values. Available online: https://www.ghgprotocol.org/sites/default/files/ghgp/Global-Warming-Potential-Values%20%28Feb%2016%202016%29_1.pdf (accessed on 10 September 2019).
- IPCC, Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: New York, NY, USA, 2014.
- IPCC—Intergovernmental Panel on Climate Change. Climate Change 2014 Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IPCC—Intergovernmental Panel on Climate Change. Global warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018; ISBN 9789291691517. [Google Scholar]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage–A review. Petroleum 2019, 5, 335–340. [Google Scholar] [CrossRef]
- Wilberforce, T.; Baroutaji, A.; Soudan, B.; Al-alami, A.H.; Ghani, A. Science of the Total Environment Outlook of carbon capture technology and challenges. Sci. Total Environ. 2019, 657, 56–72. [Google Scholar] [CrossRef] [Green Version]
- Sreedhar, I.; Nahar, T.; Venugopal, A.; Srinivas, B. Carbon capture by absorption–Path covered and ahead. Renew. Sustain. Energy Rev. 2017, 76, 1080–1107. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Miller, D.C. Post-combustion CO2 capture technologies—A review of processes for solvent-based and sorbent-based CO2 capture. Curr. Opin. Chem. Eng. 2017, 17, 78–92. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. International Journal of Greenhouse Gas Control A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Naraharisetti, P.K.; Yeo, T.Y.; Bu, J. New classification of CO2 mineralization processes and economic evaluation. Renew. Sustain. Energy Rev. 2019, 99, 220–233. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Sreedhar, I.; Vaidhiswaran, R.; Kamani, B.M.; Venugopal, A. Process and engineering trends in membrane based carbon capture. Renew. Sustain. Energy Rev. 2017, 68, 659–684. [Google Scholar] [CrossRef]
- Xu, J.; Wu, H.; Wang, Z.; Qiao, Z.; Zhao, S.; Wang, J. Chinese Journal of Chemical Engineering Recent advances on the membrane processes for CO2 separation. Chinese J. Chem. Eng. 2018, 26, 2280–2291. [Google Scholar] [CrossRef]
- Han, Y.; Ho, W.S. Recent advances in polymeric membranes for CO2 capture. Chinese J. Chem. Eng. 2018, 26, 2238–2254. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhao, S.; Wang, J.; Wang, S. Recent advances on mixed matrix membranes for CO2 separation. Chinese J. Chem. Eng. 2017, 25, 1581–1597. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Chung, T.; Ying, L.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Ebadi, A. Progress in Polymer Science State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Venturi, D.; Chrysanthou, A.; Dhuiège, B.; Missoum, K.; Baschetti, M.G. Arginine/Nanocellulose membranes for carbon capture applications. Nanomaterials 2019, 9, 877. [Google Scholar] [CrossRef] [Green Version]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Enhancing CO2 separation performance of Pebax® MH-1657 with aromatic carboxylic acids. Sep. Purif. Technol. 2019, 212, 901–912. [Google Scholar] [CrossRef]
- Lee, S.; Chan, S.; Kim, T.; Wook, S.; Soo, Y. Direct molecular interaction of CO2 with KTFSI dissolved in Pebax 2533 and their use in facilitated CO2 transport membranes. J. Memb. Sci. 2018, 548, 358–362. [Google Scholar] [CrossRef]
- Eun, J.; Ki, S.; Hoon, Y.; Bum, H. Effect of PEG-MEA and graphene oxide additives on the performance of Pebax®1657 mixed matrix membranes for CO2 separation. J. Memb. Sci. 2019, 572, 300–308. [Google Scholar]
- Zheng, W.; Ding, R.; Yang, K.; Dai, Y.; Yan, X.; He, G. ZIF-8 nanoparticles with tunable size for enhanced CO2 capture of Pebax based MMMs. Sep. Purif. Technol. 2019, 214, 111–119. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Memb. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Arkema ARKEMA Products Online Database. Available online: https://www.extremematerials-arkema.com/en/materials-database/products (accessed on 23 October 2019).
- Deluca, N. PEBA: TPE materials for high performance applications. In Proceedings of the Annual Technical Conference - ANTEC, Anaheim, CA, USA, 8–10 May 2017; pp. 2343–2393. [Google Scholar]
- Sheth, J.P.; Xu, J.; Wilkes, G.L. Solid state structure–property behavior of semicrystalline poly (ether-block-amide) PEBAX® thermoplastic elastomers. Polymer (Guildf.) 2003, 44, 743–756. [Google Scholar] [CrossRef]
- Arkema, Arkema Reference Document 2018 Including the Annual Financial Report. Labrador Information Design: Atlanta, GA, USA, 2019; pp. 1–364.
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Memb. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y. Metal-organic framework-graphene oxide composites: A facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Memb. Sci. 2016, 520, 801–811. [Google Scholar] [CrossRef]
- Scofield, J.M.P.; Gurr, P.A.; Kim, J.; Fu, Q.; Kentish, S.E.; Qiao, G.G. Development of novel fluorinated additives for high performance CO2 separation thin-film composite membranes. J. Memb. Sci. 2016, 499, 191–200. [Google Scholar] [CrossRef]
- Liu, Y.C.; Chen, C.Y.; Lin, G.S.; Chen, C.H.; Wu, K.C.W.; Lin, C.H.; Tung, K.L. Characterization and molecular simulation of Pebax-1657-based mixed matrix membranes incorporating MoS2 nanosheets for carbon dioxide capture enhancement. J. Memb. Sci. 2019, 582, 358–366. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Ahmadi, R.; Ebadi Amooghin, A.; Ghanbari, D. A novel ternary mixed matrix membrane containing glycerol-modified poly(ether-block-amide) (Pebax 1657)/copper nanoparticles for CO2 separation. J. Memb. Sci. 2019, 573, 234–246. [Google Scholar] [CrossRef]
- Wang, J.; Fang, W.; Luo, J.; Gao, M.; Wan, Y.; Zhang, S.; Zhang, X.; Park, A.H.A. Selective separation of CO2 using novel mixed matrix membranes based on Pebax and liquid-like nanoparticle organic hybrid materials. J. Memb. Sci. 2019, 584, 79–88. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.B. Development of nanocomposite membranes containing modified Si nanoparticles in PEBAX-2533 as a block copolymer and 6FDADurene diamine as a glassy polymer. ACS Appl. Mater. Interfaces 2014, 6, 15643–15652. [Google Scholar] [CrossRef] [PubMed]
- Koolivand, H.; Razzaghi-kashani, M.; Karimi, M.; Koolivand, M. Functionalized graphene oxide/polyimide nanocomposites as highly CO2-selective membranes. J. Polym. Res. 2014, 21, 1–12. [Google Scholar] [CrossRef]
- Chen, M.; Soyekwo, F.; Zhang, Q.; Hu, C.; Zhu, A.; Liu, Q. Graphene oxide nanosheets to improve permeability and selectivity of PIM-1 membrane for carbon dioxide separation. J. Ind. Eng. Chem. 2018, 63, 296–302. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Li, S.; Yu, M. Ultrathin graphene oxide-based hollow fiber membranes with brush-like CO2-philic agent for highly efficient CO2 capture. Nat. Commun. 2017, 8, 1–8. [Google Scholar]
- Dong, G.; Hou, J.; Wang, J.; Zhang, Y.; Chen, V.; Liu, J. Enhanced CO2/N2 separation by porous reduced graphene oxide / Pebax mixed matrix membranes. J. Memb. Sci. 2016, 520, 860–868. [Google Scholar] [CrossRef]
- Wang, D.; Yao, D.; Wang, Y.; Wang, F.; Xin, Y.; Song, S.; Zhang, Z.; Su, F.; Zheng, Y. Carbon nanotubes and graphene oxide-based solvent-free hybrid nanofluids functionalized mixed-matrix membranes for efficient CO2/N2separation. Sep. Purif. Technol. 2019, 221, 421–432. [Google Scholar] [CrossRef]
- Anastasiou, S.; Bhoria, N.; Pokhrel, J.; Reddy, K.S.K.; Srinivasakannan, C.; Wang, K.; Karanikolos, G.N. Metal-organic framework / graphene oxide composite fillers in mixed-matrix membranes for CO2 separation. Mater. Chem. Phys. 2018, 212, 513–522. [Google Scholar] [CrossRef]
- Mohammed, S.A.; Nasir, A.M.; Aziz, F.; Kumar, G.; Sallehhudin, W.; Jaafar, J.; Lau, W.J.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F. CO2/N2 selectivity enhancement of PEBAX MH 1657/Aminated Partially Reduced Graphene Oxide Mixed Matrix Composite Membrane. Sep. Purif. Technol. 2019. [Google Scholar] [CrossRef]
- Janakiram, S.; Ahmadi, M.; Dai, Z.; Ansaloni, L.; Deng, L. Performance of nanocomposite membranes containing 0D to 2D nanofillers for CO2 separation: A review. Membranes 2018, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Sun, M.; Li, J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today 2018, 20, 121–137. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.V. CO2-selective PEO-PBT (PolyActiveTM)/graphene oxide composite membranes. Chem. Commun. 2015, 51, 14187–14190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Liu, G.; Huang, K.; Jin, W.; Lee, K.R.; Xu, N. Membranes with fast and selective gas-transport channels of laminar graphene oxide for efficient CO2 capture. Angew. Chemie Int. Ed. 2015, 54, 578–582. [Google Scholar]
- Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Zhai, H.; Rubin, E. Membrane properties required for post-combustion CO2 capture at coal-fired power plants. J. Memb. Sci. 2016, 511, 250–264. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, B.; Yan, F.; Zhao, J.; Li, J.; Li, L. Engineering nano-porous graphene oxide by hydroxyl radicals. Carbon N. Y. 2019, 105, 291–296. [Google Scholar] [CrossRef]
- Yoo, M.J.; Kim, H.W.; Yoo, B.M.; Park, H.B. Highly soluble polyetheramine-functionalized graphene oxide and reduced graphene oxide both in aqueous and non-aqueous solvents. Carbon N. Y. 2014, 75, 149–160. [Google Scholar] [CrossRef]
- Lara-estévez, J.C.I.; Antônio, L.; de Almeida, S.; Schulte, K.; Bucio, E. PEBAX TM-Silanized Al2O3 Composite, Synthesis and Characterization. Open J. Polym. Chem. 2012, 2, 63–69. [Google Scholar]
- Armstrong, S.; Freeman, B.; Hiltner, A.; Baer, E. Gas permeability of melt-processed poly (ether block amide) copolymers and the effects of orientation. Polymer (Guildf.) 2012, 53, 1383–1392. [Google Scholar] [CrossRef]
- Liu, K.; Fang, C.; Li, Z.; Young, M. Separation of thiophene/n-heptane mixtures using PEBAX/PVDF-composited membranes via pervaporation. J. Memb. Sci. 2014, 451, 24–31. [Google Scholar] [CrossRef]
- Clarizia, G.; Bernardo, P.; Gorrasi, G.; Zampino, D.; Carroccio, S.C. Influence of the Preparation Method and Mhoto-Oxidation Treatment on the Thermal and Gas Transport Properties of Dense Films Based on a Poly(ether-block-amide) Copolymer. Materials 2018, 11, 1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahab, M.S.A.; Sunarti, A.R. Development of PEBAX Based Membrane for Gas Separation: A Review. Int. J. Membr. Sci. Technol. 2015, 2, 78–84. [Google Scholar]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Lee, H.D.; Shin, J.E.; Yoo, B.M.; Park, H.B. Water and ion sorption, diffusion, and transport in graphene oxide membranes revisited. J. Memb. Sci. 2017, 544, 425–435. [Google Scholar] [CrossRef]
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Memb. Sci. 2020, 603, 118019. [Google Scholar] [CrossRef]
- Su, Y.; University of Manchester; Wilkinson, J.; AzoNano. Interview: Developing Graphene Oxide Membranes for the Purification of Water and Green Fuels. Available online: https://www.azonano.com/article.aspx?ArticleID=4275 (accessed on 14 May 2020).
- Ehsani, A.; Pakizeh, M. Journal of the Taiwan Institute of Chemical Engineers Synthesis, characterization and gas permeation study of ZIF-11 / Pebax ® 2533 mixed matrix membranes. J. Taiwan Inst. Chem. Eng. 2016, 66, 414–423. [Google Scholar] [CrossRef]
- Cho, Y.H.; Jeong, S.M.; Kim, S.; Kim, Y.; Lee, H.J.; Lee, T.H.; Park, H.B.; Park, H.; Nam, S.; Park, Y. Sacrificial graphene oxide interlayer for highly permeable ceramic thin film composite membranes. J. Memb. Sci. 2020, 118442. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.C.; Roh, J.S.; Moon, G.H.; Shin, J.E.; Kang, Y.S.; Park, H.B. Metal-organic frameworks grown on a porous planar template with an exceptionally high surface area: Promising nanofiller platforms for CO2 separation. J. Mater. Chem. A 2017, 5, 22500–22505. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Li, N.; Liao, J.; Zhao, S.; Wang, J.; Wang, S. Relationship between polymer-filler interfaces in separation layers and gas transport properties of mixed matrix composite membranes. J. Memb. Sci. 2015, 495, 252–268. [Google Scholar] [CrossRef]
- Takahashi, S.; Paul, D.R. Gas permeation in poly(ether imide) nanocomposite membranes based on surface-treated silica. Part 1: Without chemical coupling to matrix. Polymer (Guildf.) 2006, 47, 7519–7534. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic-inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive models for mixed-matrix membrane performance: A review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef] [PubMed]
- Baig, Z.; Mamat, O.; Mustapha, M.; Mumtaz, A.; Munir, K.S.; Sarfraz, M. Investigation of tip sonication effects on structural quality of graphene nanoplatelets (GNPs) for superior solvent dispersion. Ultrason. Sonochem. 2018, 45, 133–149. [Google Scholar] [CrossRef]















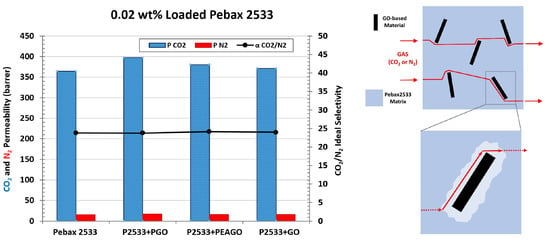
| Membrane | CO2 Permeability (Barrer) | CO2 Permeability Variation% | N2 Permeability (Barrer) | N2 Permeability Variation% | CO2/N2 Selectivity | CO2/N2 Variation% |
|---|---|---|---|---|---|---|
| Pebax 2533 | 364.61 | \ | 15.32 | \ | 23.80 | \ |
| +0.02% GO | 371.39 | 1.86 | 15.47 | 1.01 | 24.00 | 0.84 |
| +0.1% GO | 336.80 | −7.63 | 14.02 | −8.47 | 24.02 | 0.92 |
| +0.5% GO | 100.61 | −72.41 | 4.19 | −72.65 | 24.01 | 0.88 |
| +1% GO | 48.58 | −86.68 | 2.17 | −85.83 | 22.38 | −5.97 |
| +0.02% PGO | 397.35 | 8.98 | 16.73 | 9.19 | 23.75 | −0.19 |
| +0.02% PEAGO | 380.44 | 4.34 | 15.73 | 2.65 | 24.19 | 1.64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casadei, R.; Giacinti Baschetti, M.; Yoo, M.J.; Park, H.B.; Giorgini, L. Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture. Membranes 2020, 10, 188. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10080188
Casadei R, Giacinti Baschetti M, Yoo MJ, Park HB, Giorgini L. Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture. Membranes. 2020; 10(8):188. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10080188
Chicago/Turabian StyleCasadei, Riccardo, Marco Giacinti Baschetti, Myung Jin Yoo, Ho Bum Park, and Loris Giorgini. 2020. "Pebax® 2533/Graphene Oxide Nanocomposite Membranes for Carbon Capture" Membranes 10, no. 8: 188. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10080188