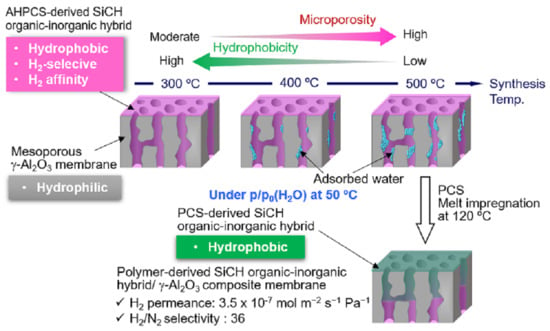
3.3. Properties of SiCH Hybrid/γ-Al2O3 Composite Membrane
- (1)
Structure of the composite membrane
As a typical observation,
Figure 3a presents a cross-sectional SEM image of a supported γ-Al
2O
3 membrane after dip coating of the AHPCS xylene solution and subsequent heat treatment at 400 °C under flowing Ar. There was no additional layer on the γ-Al
2O
3 membrane surface. Then, an EDS analysis was performed on the modified membrane. As shown in
Figure 3b, the line scan of the EDS mapping for Si derived form AHPCS was detected within the γ-Al
2O
3 membrane having approximately 2.5 μm thickness. Accordingly, the resulting composite membranes in this study were composed of γ-Al
2O
3 with mesopore channels infiltrated by the AHPCS-derived SiCH hybrid.
- (2)
Gas permeation behaviors under dry condition
Arrhenius plots of He, H
2 and N
2 permeances evaluated for the SiCH hybrid/γ-Al
2O
3 composite membranes synthesized at 300, 400 and 500 °C are shown in
Figure 4a–c, respectively. Despite the low permeation temperature of 25 °C, the composite membranes exhibited a relatively high H
2 permeance of 1 × 10
−7 to 4 × 10
−6 mol m
−2 s
−1 Pa
−1 with a H
2/N
2 permselectivity (α(H
2/N
2)) of 9.2–17, apparently higher than that of the theoretical one (3.73) based on the Knudsen’s diffusion.
The gas permeation behavior through each composite membrane was similar, and all the gas permeances increased linearly with the permeation temperature and thus followed the Arrhenius law. Since the gas permeations through the supported mesoporous γ-Al
2O
3 membrane exhibited a typical Knudsen’s diffusion characteristics in our previous study [
50], the gas permeation behaviors observed for the present composite membranes suggested that all the gases permeated through the microporous SiCH hybrid, which filled in the mesopore channels of the γ-Al
2O
3, and the dominant mechanism for the gas permeations was activated diffusion. However, at all permeation temperatures from 25 to 80 °C, the composite membranes exhibited a unique α(H
2/He) of 1.44–1.95, which was higher than the theoretical one (1.41) based on the Knudsen’s diffusion.
To highlight this unique gas permeation behavior, the gas permeances measured at 50 °C were characterized and are shown in
Figure 5 by plotting the kinetic diameter dependence of the normalized gas (
i) permeance relative to the He permeance (
Qi/
QHe), and compared with the ideal value (
QK,i/
QK,He) by the Knudsen model [
63,
64],
where
Mi and
MHe are molecular weights of gas-
i and He, respectively.
The apparent activation energies for He and H
2 permeations were evaluated by the Arrhenius plot (
Figure 4) and are listed in
Table 1. The apparent activation energy (
Ea) for He permeation was 20.3–1.4 kJ mol
−1, while that for H
2 permeation was 17.0–0.5 kJ mol
−1.
The
Ea values for both He and H
2 permeations decreased with the composite membrane synthesis temperature. This tendency was well consistent with the result of TG-MS analyses (
Figures S3 and S4) and the gas permeation behaviors (
Figure 4): weight loss due to the volatilization of the low molecular weight fraction of as-received AHPCS contentiously proceeded at 300–500 °C (
Figure S4), and resulting yields of the SiCH hybrid at 300, 400 and 500 °C were measured to be 87%, 83% and 76%, respectively (
Figure S3), while all the gas permeances through the composite membrane increased with the synthesis temperature (
Figure 4). Thus, the observed
Ea values for He and H
2 permeations could depend on the density of the AHPCS-derived SiCH organic–inorganic hybrid network. However, a unique point was that, regardless of the synthesis temperature, the
Ea for H
2 permeation was found to be smaller than that for He permeation, which was a clear contrast to those evaluated for the H
2-selecitve microporous amorphous silica membranes [
10,
11,
65,
66,
67]. Then, an attempt was made for study on the hydrogen affinity of the AHPCS-derived SiCH organic–inorganic hybrid: The amount of H
2 adsorption on the AHPCS-derived SiCH film was measured by using a quartz-crystal microbalance (QCM). The SiCH sample film was formed on the quartz crystal unit surface by following the procedure for the composite membrane synthesis at 300 °C (effective film area corresponded to
Ae in Equation (2), 3.93 × 10
−5 m
2). The sample film was exposed to He atmosphere under the strictly regulated isothermal condition at 30 °C (±0.1 °C) for more than 90 ks. After the He adsorption reached equilibrium, weight gain was monitored by exposing the sample film to H
2 atmosphere under the same isothermal condition. As shown in
Figure 6, the weight gain increased with H
2 exposure time and reached 7.14 ng (1.82 × 10
−4 g m
−2) after the additional 88,130 s. Since the molecular weight of H
2 (2.016) was approximately one-half of He (4.002), the weight gain measured under the H
2 atmosphere revealed preferential adsorption of H
2 relative to He, i.e., existence of H
2 affinity of the AHPCS-derived SiCH organic–inorganic hybrid, which might contribute to the experimentally observed unique α(H
2/He) > 1.41 and high H
2 permeances (10
−7–10
−6 mol m
−2 s
−1 Pa
−1 order at 25–80 °C).
- (3)
Gas permeation behaviors under the wet condition
For study on the potential application to the solar hydrogen production system, the gas permeation measurement was performed on the composite membranes by using a mixed H
2-N
2 feed gas in the molar ratio 2:1 under dry and saturated water vapor partial pressure (p/p
0 (H
2O) = 1) at 50 °C. The results are summarized and shown in
Figure 7. The composite membranes were found to be water vapor permeable and the permeance was measured to be 2.7–3.8 × 10
−7 mol m
−2 s
−1 Pa
−1.
The α(H2/N2) of the composite membranes was 6.0–11.3, and regardless of the synthesis temperature, there was no significant degradation under the highly humid condition at 50 °C. The 300 °C-synthesized composite membrane kept relatively high gas permeances. The retentions evaluated for the H2 and N2 permeances under the p/p0 (H2O) = 1 at 50 °C were 69 and 67%, respectively. However, with increasing synthesis temperature, the permeance retention of both H2 and N2 decreased, in other words, the hydrophobicity in terms of stable gas permeation property degraded with the synthesis temperature. This degradation tendency is consistent with the temperature dependence of the SiCH hybrid polymer/highly cross-linked SiCH hybrid conversion yield as described above, and thus is related to the quantity of organic groups remained in the SiCH hybrid and the microporosity or volume of the SiCH hybrid, which infiltrated the mesopore channels of the composite membrane.
Then, the 500 °C-synthesized composite membrane was further modified with polycarbosilane (PCS) by the 120 °C-melt impregnation established by our previous study [
50]. As shown in
Figure 7, under the dry condition at 50 °C, the PCS-modified composite membrane showed a relatively high H
2 permeance of 8.4 × 10
−7 mol m
−2 s
−1 Pa
−1 with a significantly improved α(H
2/N
2) of 29.6. Moreover, under the p/p
0 (H
2O) = 1 at 50 °C, the PCS-modification successfully improved the membrane performance: H
2 permeance and α(H
2/N
2) were measured to be 3.5 × 10
−7 mol m
−2 s
−1 Pa
−1 and 36, respectively.
Gas permeation properties under the wet condition at 50 °C of the composite membranes were also assessed by cyclic gas permeance measurements under p/p
0(H
2O) ranging from 0.1 to 1.0 and compared with those of the supported mesoporous γ-Al
2O
3 membrane itself. As shown in
Figure 8a, H
2 and N
2 permeances through the supported mesoporous γ-Al
2O
3 membrane drastically decreased above p/p
0(H
2O) = 0.74. This degradation is due to the highly hydrophilic property of γ-Al
2O
3, which leads to the blockage of the gas permeable mesopore channels by adsorption and subsequent condensation of water molecules as the permeate [
50]. On the other hand, the 300 °C-synthesized composite membrane exhibited stable gas permeations at all the p/p
0(H
2O) up to 1.0 (
Figure 8b). A slight decrease in the gas permeances at p/p
0(H
2O) > 0.74 was due to the pressure-drop caused by the water vapor condensation within the mesopore channels of hydrophilic γ-Al
2O
3 partly remained without surface modification with the AHPCS-derived hydrophobic hybrid. The 500 °C-synthesized composite membrane also showed the decreasing tendency in gas permeances, however further modification with PCS successfully improved the membrane performance at the final gas permeation measurement under the saturated humidity at 50 °C: H
2 permeance remained at 10
−7 mol m
−2 s
−1 Pa
−1 order with α(H
2/N
2) > 30 under (
Figure 8c), and the resulting separation factor (SF) evaluated based on the gas permeation data shown in
Table 2 was found as 26.
Moreover, the polymer-derived SiCH organic–inorganic hybrid investigated in this study showed sufficient stability under the present high humidity conditions at 50 °C:
Figure 9 presents the top surface view of the 500 °C-synthesized composite membrane modified with PCS before and after the cyclic gas permeation measurements under p/p
0(H
2O) up to 1.0 at 50 °C. Compared with the surface of the as-synthesized mesoporous γ-Al
2O
3 membrane over an α-Al
2O
3 porous support (
Figure 9a), the composite membrane exhibited a smooth surface (
Figure 9b) and kept the surface without structural degradation after the cyclic gas permeation measurements (
Figure 9c). These results revealed that, in addition to hydrogen permselectivity, the modification with the polymer-derived SiCH organic–inorganic hybrid investigated in this study greatly improved the hydrophobicity in terms of stable gas permeations under the saturated water vapor partial pressure at 50 °C.
The H
2-permselectivities of the composite membrane measured in this study were briefly compared with those recently reported for other membranes composed of various materials systems, and their H
2 permeation data with α(H
2/X) (X = N
2 (0.364 nm) [
51] or O
2 (0.346 nm) [
51]) measured under the dry condition at
T ≤ 50 °C are listed in
Table 3 [
18,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78]. Among them, novel ultrathin (9 nm thickness) graphene oxide membrane formed on an anodic oxidized alumina support (#09) exhibited a H
2 permeance of approximately 1 × 10
−7 mol m
−2 s
−1 Pa
−1 with α(H
2/N
2) of 900 at 20 °C [
75,
76]. The zeolite imidazolate framework (ZIF) nanosheet membrane (#08) also showed H
2 permeance of 2.04 × 10
−7 mol m
−2 s
−1 Pa
−1 with high α(H
2/N
2) of 66.6 at 30 °C [
73,
74]. Among other practical membranes, SiO
2-based organic–inorganic hybrid membrane (#03) showed the highest H
2 permeance of 10
−6 mol m
−2 s
−1 Pa
−1 order, while the α(H
2/N
2) remained at 12 [
68]. On the other hand, zeolite/CMC (carbon molecular sieve) composite membranes (#04, #05) showed a high α(H
2/N
2) of 61-100.2, however the H
2 permeance at 30 °C was 10
−9–10
−8 mol m
−2 s
−1 Pa
−1 order [
69,
70].
As shown in
Figure 4 and
Figure 5, in addition to the H
2/N
2 selectivity, the present composite membranes exhibited unique H
2/He selectivity due to the H
2 affinity of the AHPCS-derived highly cross-linked SiCH organic–inorganic hybrid in the composite membrane (
Figure 6). This unique H
2 preferential permeation property contributed to relatively high H
2 permeance of 8.4 × 10
−7 mol m
−2 s
−1 Pa
−1 with α(H
2/N
2) of 29.6.
For the application of the purification of solar hydrogen, the long-term stability and robustness of H
2-selecitve membranes under the humid condition at around 50 °C are practical issues for us to pursue. In this context, there are few reports as mentioned above, and Oyama et al. reported [
18] their pioneering study on the stability of PFDA-based liquid membrane (#06 in
Table 3) under the humid condition (10 mol%) at 30 °C as a simulated condition for the purification of solar hydrogen, and they confirmed its stability for up to 48 h. The stabilities of the composited membranes characterized by the primary accelerated degradation test (
Figure 7,
Figure 8 and
Figure 9) were compatible with that of the supported liquid membrane (#06 in
Table 3) [
18]. Under the scheme of the current NEDO R&D “Artificial Photosynthesis” Project, we plan to conduct the long-term stability test for the composite membranes by using a H
2-O
2 (2:1) mixed feed gas as a simulated syngas at the project facility with safety measures against explosion.