Amino Acid Cross-Linked Graphene Oxide Membranes for Metal Ions Permeation, Insertion and Antibacterial Properties
Abstract
:1. Introduction
2. Experimentals
2.1. Regents and Materials
2.2. Preparation and Characterizations of GOMs
2.3. Measurement of Water Fluxes, and Test of Stability and Tensile Properties
2.4. Stability and Tensile Properties of GOMs
2.5. Permeation Experiment
2.6. GO-PHE Membrane Insertion Experiment
2.7. Antibacterial Ability Measurement
3. Results and Discussion
3.1. Characterization of GO-Ala, GO-PHE, GO-Ser
3.2. Contact Angle, Water Flux, Stability and Tensile Properties of GOM, GO-Ala, GO-PHE, and GO-Ser
3.3. Rejection Coefficients of GO-Ala, GO-PHE, and GO-Ser
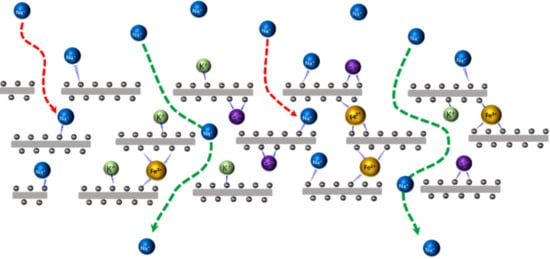
3.4. Permeability of Na+ Through GO-PHE-Inserted K+, Ca2+, and Fe3+
3.5. Antibacterial Performance of GOM, GO-Ala, GO-PHE, and GO-Ser
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Chang, H.; Liang, H.; Qu, F.; Liu, B.; Yu, H.; Du, X.; Li, G.; Snyder, S.A. Hydraulic backwashing for low-pressure membranes in drinking water treatment: A review. J. Membr. Sci. 2017, 540, 362–380. [Google Scholar] [CrossRef]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- She, Q.; Chi, L.; Zhou, W.; Zhang, Z. Overview of forward osmosis membrane separation technology: Research and its application to water treatment. Environ. Sci. Technol. 2010, 33, 117–122. [Google Scholar]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- O’Hern, S.C.; Jang, D.; Bose, S.; Idrobo, J.-C.; Song, Y.; Laoui, T.; Kong, J.; Karnik, R. Nanofiltration across Defect-Sealed Nanoporous Monolayer Graphene. Nano Lett. 2015, 15, 3254–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Liu, Y.; Zhao, Y.; Zhang, X.; Qian, L.; Tian, L.; Bai, J.; Qi, W.; Yao, H.; Gao, B.; et al. Selective Separation of Metal Ions via Monolayer Nanoporous Graphene with Carboxyl Groups. Anal. Chem. 2016, 88, 10002–10010. [Google Scholar] [CrossRef]
- Yang, Q.; Su, Y.; Chi, C.; Cherian, C.T.; Huang, K.; Kravets, V.G.; Wang, F.C.; Zhang, J.C.; Pratt, A.; Grigorenko, A.N.; et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 2017, 16, 1198–1202. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Xu, W.L.; Zhou, F.; Yoon, Y.; Yu, M. Graphene Oxide: A Novel 2-Dimensional Material in Membrane Separation for Water Purification. Adv. Mater. Interfaces 2017, 4, 1600918. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Guan, K.; Liu, G.; Jin, W. Cysteamine-crosslinked graphene oxide membrane with enhanced hydrogen separation property. J. Membr. Sci. 2020, 595, 117568. [Google Scholar] [CrossRef]
- Lim, M.-Y.; Choi, Y.-S.; Kim, J.; Kim, K.; Shin, H.; Kim, J.-J.; Shin, D.M.; Lee, J.-C. Cross-linked graphene oxide membrane having high ion selectivity and antibacterial activity prepared using tannic acid-functionalized graphene oxide and polyethyleneimine. J. Membr. Sci. 2017, 521, 1–9. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Z.; Zheng, S.; Mi, B. Polyamide-crosslinked graphene oxide membrane for forward osmosis. J. Membr. Sci. 2018, 545, 11–18. [Google Scholar] [CrossRef]
- Liu, T.; Yang, B.; Graham, N.; Yu, W.; Sun, K. Trivalent metal cation cross-linked graphene oxide membranes for NOM removal in water treatment. J. Membr. Sci. 2017, 542, 31–40. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Shi, W. Tailoring permeation channels of graphene oxide membranes for precise ion separation. Carbon 2016, 101, 290–295. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, L.; Chen, H.; Gao, C. Sol–gel fabrication of a non-laminated graphene oxide membrane for oil/water separation. J. Mater. Chem. A 2015, 3, 19517–19524. [Google Scholar] [CrossRef]
- Wang, J.; Huang, T.; Zhang, L.; Yu, Q.J.; Hou, L. Dopamine crosslinked graphene oxide membrane for simultaneous removal of organic pollutants and trace heavy metals from aqueous solution. Environ. Technol. 2018, 39, 3055–3065. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, W.; Liu, Y.; Qin, C.; Meng, M.; Jiang, Y.; Qiu, J.; Peng, J. A mussel inspired highly stable graphene oxide membrane for efficient oil-in-water emulsions separation. Sep. Purif. Technol. 2018, 199, 37–46. [Google Scholar] [CrossRef]
- Xia, S.; Ni, M.; Zhu, T.; Zhao, Y.; Li, N. Ultrathin graphene oxide nanosheet membranes with various d-spacing assembled using the pressure-assisted filtration method for removing natural organic matter. Desalination 2015, 371, 78–87. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Yan, F. Fabrication of Graphene Oxide forward Osmosis Membrane and Its Separation Performance. Ph.D. Dissertation, Chinese Academy of Science, Beijing, China, 2017. [Google Scholar]
- Cheng, P.; Chen, Y.; Yan, X.; Wang, Y.; Lang, W.-Z. Ultrastable and antibacterial two-dimension tungsten disulfide lamellar membrane for water filtration. Chemsuschem 2018, 12, 275–282. [Google Scholar] [CrossRef]
- Ma, F.; Li, Z.; Zhao, H.; Geng, Y.; Zhou, W.; Li, Q.; Zhang, L. Potential application of graphene oxide membranes for removal of Cs(I) and Sr(II) from high level-liquid waste. Sep. Purif. Technol. 2017, 188, 523–529. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabó, T.; Szeri, A.; Dékány, I. Graphite Oxide: Chemical Reduction to Graphite and Surface Modification with Primary Aliphatic Amines and Amino Acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Mikulchyk, T.; Walshe, J.; Cody, D.; Martin, S.; Naydenova, I. Humidity and temperature induced changes in the diffraction efficiency and the Bragg angle of slanted photopolymer-based holographic gratings. Sens. Actuators B-Chem. 2017, 239, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-linking with diamine monomers to prepare composite graphene oxide-framework membranes with varying d-spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Choi, W.; Chun, K.-Y.; Kim, J.; Han, C.-S. Ion transport through thermally reduced and mechanically stretched graphene oxide membrane. Carbon 2017, 114, 377–382. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Lee, H.D.; Shin, J.E.; Yoo, B.M.; Park, H.B. Water and ion sorption, diffusion, and transport in graphene oxide membranes revisited. J. Membr. Sci. 2017, 544, 425–435. [Google Scholar] [CrossRef]
- Park, S.; Dikin, D.A.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Sheets Chemically Cross-Linked by Polyallylamine. J. Phys. Chem. C 2009, 113, 15801–15804. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA 300 Database. John Willey: Chichester, UK, 1992. [Google Scholar]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Deng, C.-H.; Yang, H.-C.; Liu, H.-Y.; Huan, S.-Y. Cross-linking to prepare composite graphene oxide-framework membranes with high-flux for dyes and heavy metal ions removal. Chem. Eng. J. 2017, 322, 657–666. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Lee, K.H.; Chu, J.Y.; Ryu, S.K.; Kim, H.G.; Lee, J.Y.; Lee, H.K.; Yoo, D.J. Ameliorated Performance of Sulfonated Poly(Arylene Ether Sulfone) Block Copolymers with Increased Hydrophilic Oligomer Ratio in Proton-Exchange Membrane Fuel Cells Operating at 80% Relative Humidity. Polymers 2020, 12, 1871. [Google Scholar] [CrossRef]
- Chen, L.; Moon, J.-H.; Ma, X.; Zhang, L.; Chen, Q.; Chen, L.; Peng, R.; Si, P.; Feng, J.; Li, Y.; et al. High performance graphene oxide nanofiltration membrane prepared by electrospraying for wastewater purification. Carbon 2018, 130, 487–494. [Google Scholar] [CrossRef]
- Hu, P.; Huang, B.; Miao, Q.; Wang, H.; Liu, L.; Tai, W.; Liu, T.; Li, Z.; Chen, S.; Qian, L. Ion transport behavior through thermally reduced graphene oxide membrane for precise ion separation. Crystals 2019, 9, 214. [Google Scholar] [CrossRef] [Green Version]
- Volkov, A.G.; Paula, S.; Deamer, D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997, 42, 153–160. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.; Cao, K.; Li, B.; Li, Y.; Zhang, M.; Wen, R.; Li, X.; Li, S.; Ma, L. Effective charge-discriminated group separation of metal ions under highly acidic conditions using nanodiamond-pillared graphene oxide membrane. J. Mater. Chem. A 2017, 5, 8051–8061. [Google Scholar] [CrossRef]
- Yu, H.; He, Y.; Xiao, G.; Fan, Y.; Ma, J.; Gao, Y.; Hou, R.; Yin, X.; Wang, Y.; Mei, X. The roles of oxygen-containing functional groups in modulating water purification performance of graphene oxide-based membrane. Chem. Eng. J. 2020, 389, 124375. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]










| Membrane | Contact Angle (°) | Water Flux (L m−2 h−1) | Water Phase * (s) | Tensile Properties (MPa) |
|---|---|---|---|---|
| GOM GO-Ala | 52 44.4 | 6.07 4.72 | 14 54 | 27.2 26.4 |
| GO-PHE | 53.8 | 6.61 | 36 | 25.8 |
| GO-Ser | 53.2 | 11.81 | 43 | 27.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, L.; Wang, H.; Yang, J.; Chen, X.; Chang, X.; Nan, Y.; He, Z.; Hu, P.; Wu, W.; Liu, T. Amino Acid Cross-Linked Graphene Oxide Membranes for Metal Ions Permeation, Insertion and Antibacterial Properties. Membranes 2020, 10, 296. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10100296
Qian L, Wang H, Yang J, Chen X, Chang X, Nan Y, He Z, Hu P, Wu W, Liu T. Amino Acid Cross-Linked Graphene Oxide Membranes for Metal Ions Permeation, Insertion and Antibacterial Properties. Membranes. 2020; 10(10):296. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10100296
Chicago/Turabian StyleQian, Lijuan, Haijing Wang, Jingyi Yang, Xiaolei Chen, Xue Chang, Yu Nan, Zhuanyan He, Peizhuo Hu, Wangsuo Wu, and Tonghuan Liu. 2020. "Amino Acid Cross-Linked Graphene Oxide Membranes for Metal Ions Permeation, Insertion and Antibacterial Properties" Membranes 10, no. 10: 296. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10100296