New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Equipment
2.2. Procedure for Determination of Dissociation Constants (pKa)
2.3. Liquid-Liquid Extraction Procedure (SX)
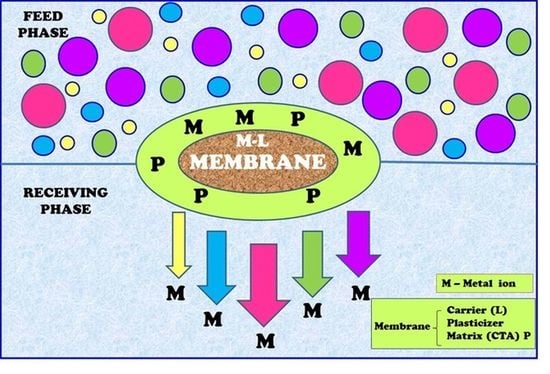
2.4. Polymer Inclusion Membrane
2.5. Transport Studies
3. Results and Discussion
3.1. Determination of Dissociation Constants (pKa)

EDAB-acac + H2O ↔ HEDAB-acac+ + OH−
HEDAB-acac+ + H2O ↔ H2EDAB-acac2+ + OH−
3.2. Solvent Extraction of Metal Ions by EDAB-acac
3.3. Determination of the Stability Constants

3.4. Transport across PIMs
3.5. Initial Fluxes, Order and Separation Coefficients for Non-Ferrous Metal Transport across PIMs
3.6. Recovery of Metal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Loon, G.W.; Duffy, S.J. Environmental Chemistry—In a Global Perspective; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Wang, L.K.; Chen, Y.-T.; Hung, N.; Shammas, K. Heavy Metals in the Environment; CRC Press, Taylor & Francis Goup: Boca Raton, FL, USA, 2009. [Google Scholar]
- Sorme, L.; Lagerkvist, R. Sources of heavy metals in urban wastewater in Stockholm. Sci. Total Environ. 2002, 298, 131–145. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Szyczewski, P.; Siepak, J.; Niedzielski, P.; Sobczynski, T. Research on heavy metals in Poland. Pol. J. Environ. Stud. 2009, 18, 755–768. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Netw. 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Radzyminska-Lenarcik, E.; Witt, K. The application of acetylacetone for the separation of heavy metals in roadside soil belts by extraction methods. Desalin. Water Treat. 2020, 186, 191–198. [Google Scholar] [CrossRef]
- Kentish, S.E.; Stevens, G.W. Innovations in separations technology for the recycling and re-use of liquid waste streams. Chem. Eng. J. 2001, 84, 149–159. [Google Scholar] [CrossRef]
- Silva, J.E.; Paiva, A.P.; Soares, D.; Labrincha, A.; Castro, F. Solvent extraction applied to the recovery of heavy metals from galvanic sludge. J. Hazard. Mat. 2005, 120, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Kislik, V.S. Solvent Extraction: Classical and Novel Approaches; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-444-53778-2. [Google Scholar]
- Zhang, J.; Hu, B. Liquid-Liquid Extraction (LLE). In Separation and Purification Technologies in Biorefineries; Ramaswamy, S., Huang, H.-J., Ramarao, B.V., Eds.; Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Alguacil, F.J. Recent trends in metal extraction. Rev. Metal. 2013, 49, 292–316. [Google Scholar] [CrossRef] [Green Version]
- Witt, K.; Radzyminska-Lenarcik, E. The recovery and the separation of metal ions from galvanic wastewaters. Desalin. Water Treat. 2018, 128, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Radzymińska-Lenarcik, E.; Ulewicz, R.; Ulewicz, M. Zinc recovery from model and waste solutions using polymer inclusion membranes (PIMs) with 1-octyl-4-methylimidazole. Desalin. Water Treat. 2018, 102, 211–219. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E. Study on effectiveness of PVC/ß-diketone sorbent in removing residue of Zn(II), Cr(III) and Ni(II) from post-galvanic wastewater. Desalin. Water Treat. 2020, 186, 199–205. [Google Scholar] [CrossRef]
- Elhalawany, N.; Baseer, R.A.; Mostafa, A.B.; Rabei, A.G. New efficient chelating polymers based on plastic waste for removal of toxic heavy metal pollutants. J. Elastom. Plast. 2017, 49, 481–497. [Google Scholar] [CrossRef]
- Cote, G. Hydrometallurgy of strategic metals. Solv. Extr. Ion Exch. 2000, 18, 703–727. [Google Scholar] [CrossRef]
- Kołtuniewicz, A.B.; Drioli, E. Membranes in Clean Technologies; Wiley-VchVerlag GmBH: Weinheim, Germany, 2008; ISBN 978-3-527-32007-3. [Google Scholar]
- Kislik, V.S. (Ed.) Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater Treatment; Elsevier: Burlington, VT, USA, 2010; ISBN 978-0-444-53218-3. [Google Scholar]
- Way, J.D.; Noble, R.D. Facilitated Transport in: Membrane Handbook; Springer Science & Business Media: New York, NY, USA, 1992. [Google Scholar]
- Zawierucha, I.; Kozłowski, C.A.; Malina, G. Removal of toxic metal ions from landfill leachate by complementary sorption and transport across polymer inclusion membranes. Waste Manag. 2013, 33, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Costache, L.N.; Szczepanski, P.; Olteanu, C.; Lica, C.G.; Teodorescu, S.; Orbeci, C. Bulk liquid membrane separation of different cations using D2EHPA and Cyanex 302 as carriers. Rev. Chim. 2014, 65, 26–32. [Google Scholar]
- De Gyves, J.; de San Miguel, E.R. Metal ion separations by supported liquid membranes. Ind. Eng. Chem. Res. 1999, 38, 2182–2202. [Google Scholar]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Zulkefeli, N.S.W.; Weng, S.K.; Halim, N.S.A. Removal of Heavy Metals by Polymer Inclusion Membranes. Curr. Pollut. Rep. 2018, 4, 84–92. [Google Scholar] [CrossRef]
- Kolev, S.D.; Almeida, M.I.G.S.; Cattrall, R.W. Polymer Inclusion Membranes, Smart Materials for Sensing and Separation. In Handbook of Smart Materials in Analytical Chemistry; de la Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Bringas, E.; Tan, N.R.; Ortiz, I.; Ghahramani, M.; Shahmirzadi, M.A.A. Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review. J. Water Proc. Eng. 2016, 9, 78–110. [Google Scholar] [CrossRef]
- Drioli, E.; Romano, M. Progress and new perspectives on integrated membrane operations for sustainable industrial growth. Ind. Eng. Chem. Res. 2001, 40, 1277–1300. [Google Scholar] [CrossRef]
- Agreda, D.D.; Garcia-Diaz, I.; López, F.A.; Alguacil, F.J. Supported liquid membranes technologies in metals removal from liquid effluents. Rev. Metal. 2011, 47, 146–168. [Google Scholar]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2018, 34, 341–363. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Staniszewski, B.; Urbaniak, W. A simple and efficient synthesis of 3-substituted derivatives of pentane-2,4-dione. Chem. Pap. 2009, 63, 212–216. [Google Scholar] [CrossRef]
- Alguacil, F.J.; Cobo, A. Solvent extraction with LIX 973N for selective separation of copper and nickel. J. Chem. Technol. Biotechnol. 1999, 74, 467–471. [Google Scholar] [CrossRef]
- Ochromowicz, K.; Jeziorek, M.; Wejman, K. Copper(II) extraction from ammonia leach solution. Physicochem. Probl. Miner. Process. 2014, 50, 327–335. [Google Scholar] [CrossRef]
- De San Miguel, E.R.; Hernández-Andaluz, A.M.; Bañuelos, J.G.; Saniger, J.M.; Aguilar, J.C.; de Gyves, J. LIX®-loaded polymer inclusion membrane for copper(II) transport: 1. Composition–performance relationships through membrane characterization and solubility diagrams. Mater. Sci. Eng. A 2006, 434, 30–38. [Google Scholar] [CrossRef]
- De Gyves, J.; Hernández-Andaluz, A.M.; de San Miguel, E.R. LIX®-loaded polymer inclusion membrane for copper (II) transport: 2. Optimization of the efficiency factors (permeability, selectivity, and stability) for LIX® 84-I. J. Membr. Sci. 2006, 268, 142–149. [Google Scholar] [CrossRef]
- Dziwinski, E.J.; Szymanowski, J. Composition of copper extractant LIX 54-100. Solv. Extr. Ion Exch. 1996, 14, 219–226. [Google Scholar] [CrossRef]
- Mickler, W.; Reich, A.; Uhlemann, E.; Bart, H.J. Liquid membrane permeation of zinc, cadmium and nickel with 4-acyl-5-pyrazolones and β-diketones. J. Membr. Sci. 1996, 119, 91–97. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Witt, K. Solvent extraction of copper ions by 3-substituted derivatives of β-diketones. Sep. Sci. Technol. 2018, 53, 1223–1229. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Urbaniak, W. Selective transport of zinc ions through a novel polymer inclusion membranes (PIMs) containing β-diketone derivatives as a carrier reagents. Sep. Sci. Technol. 2016, 51, 2620–2627. [Google Scholar] [CrossRef]
- Radzymińska-Lenarcik, E.; Witt, K.; Bożejewiecz, D. Selective transport of copper(II) ions across polymer inclusion membrane with aromatic ß-diketones as carriers. Physicochem. Probl. Miner. Process. 2018, 54, 741–750. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Ulewicz, M. Separation of Zn(II), Cr(III), and Ni(II) ions using the polymer inclusion membranes containing acetylacetone derivative as the carrier. Membranes 2020, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Böttcher, A.; Quezada, C.M.; Meade, T.J.; Gray, H.B. Inhibition of thermolysin and human -thrombin by cobalt(III) Schiff base complexes. Bioorganic Med. Chem. 1999, 7, 815–819. [Google Scholar] [CrossRef]
- Ulewicz, M.; Radzyminska-Lenarcik, E. Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes—A Review. Membranes 2020, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Radzyminska-Lenarcik, E.; Ulewicz, M. The Application of Polymer Inclusion Membranes Based on CTA with 1-alkylimidazole for the Separation of Zinc(II) and Manganese(II) Ions from Aqueous Solutions. Polymers 2019, 11, 242. [Google Scholar] [CrossRef] [Green Version]
- Braibanti, A.; Ostacoli, G.; Paoletti, P.; Pettit, L.D.; Sammartano, S. Potentiometric apparatus and technique for the pH-metric measurement of metal-complex equilibrium constants. Pure Appl. Chem. 1987, 59, 1721–1728. [Google Scholar] [CrossRef]
- Stary, J.; Liljenzin, J.O. Critical evaluation of equilibrium constants involving acetylacetone and its metal chelates. Pure Appl. Chem. 1982, 54, 2557–2592. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E. The influence of the alkyl chain length on extraction equilibrium of Cu(II) complexes with 1-alkylimidazole in aqueous solution/organic solvent system. Solv. Extr. Ion Exch. 2006, 25, 53–64. [Google Scholar] [CrossRef]
- Nicholls, D. Complexes and First-Row Transition Elements; The Macmillan Press LTD.: London, UK, 1974. [Google Scholar]
- Rzepka, M.; Kulig, J.; Lenarcik, B. Complexes of some transition cations with 7-methylpyrido [2,3-d]imidazole and 2-(2′-pyridyl)imidazole in aqueous solution. Gazz. Chim. Ital. 1992, 122, 73–77. [Google Scholar]
- Kurzak, B.; Kamecka, A.; Kurzak, K.; Jezierska, J.; Kafarski, P. Potentiometric and spectroscopic studies of the copper(II) complexes with some aminodiphosphonic acids in aqueous solution. Polyhedron 1998, 17, 4403–4413. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P. Investigation of the Stability Constants of Co(II) Complexes with a Homologous Series of 1-Alkylimidazoles in Aqueous Solution by Using a Partition Method with Several Solvents. Sep. Sci. Technol. 2004, 39, 199–226. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.; Hadjispyrou, S. The influence of solvent polarity on the tetrahedral-octahedral equilibrium of Co(II)complexes with 3,5-dimethylpyrazole. Polyhedron 1984, 3, 251–255. [Google Scholar] [CrossRef]
- Aizawa, S.; Funahashi, S. Octahedral−Tetrahedral Equilibrium and Solvent Exchange of Cobalt(II) Ions in Primary Alkylamines. Inorg. Chem. 2002, 41, 4555–4559. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E. Effect of alkyl chain length on the extraction of Cu(II) complexes with 1-alkyl-2-methylimidazole. Sep. Sci. Technol. 2007, 42, 2661–2675. [Google Scholar] [CrossRef]
- Lenarcik, B.; Ojczenasz, P.; Kopkowski, A. The Influence of the Alkyl Chain Length and Steric Effect on Stability Constants and Extractability of Co(II) Complexes with 1-Alkyl-4(5)-methylimidazoles. Sep. Sci. Technol. 2006, 41, 1697–1724. [Google Scholar] [CrossRef]
- Lenarcik, B.; Rauckyte, T. The Influence of Alkyl Length on Extraction Equilibria of Ni(II) Complexes with 1-Alkylimidazoles in Aqueous Solution/Organic Solvent Systems. Sep. Sci. Technol. 2004, 39, 3353–3372. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Chain Length and Steric Effect on Extraction of Zinc(II) Complexes with 1-Alkyl-2-methylimidazoles. Solv. Extr. Ion Exch. 2006, 24, 433–445. [Google Scholar] [CrossRef]
- Lenarcik, B.; Kierzkowska, A. The Influence of Alkyl Length on Stability Constants of Zn(II) Complexes with 1-Alkylimidazoles in Aqueous Solutions and Their Partition Between Aqueous Phase and Organic Solvent. Solv. Extr. Ion Exch. 2004, 22, 449–471. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Urbaniak, W. Cadmium(II) and lead(II) extraction and transport through polymer inclusion membranes with 1-alkylimidazole. Desalin. Water Treat. 2020, in press. [Google Scholar]
- Rydberg, J.; Musakis, C.; Choppin, G.R. Principles and Practices of Solvent Extraction; Marcel Dekker, Inc.: New York, NY, USA, 1992; Volume 1. [Google Scholar]
- Rossotti, F.J.C.; Rossotti, H. The Determination of Stability Constants; McGraw-Hill: New York, NY, USA, 1961. [Google Scholar]
- Gâzo, J.; Bersuker, I.B.; Garaj, J.; Kabešová, M.; Kohout, J.; Langfelderowá, H.; Melník, M.; Serator, M.; Valach, F. Plasticity of the coordination sphere of Copper(II) complexes, its manifestation and causes. Coord. Chem. Rev. 1976, 19, 253–297. [Google Scholar] [CrossRef]
- Danesi, P.R. Separation of metal species by supported liquid membranes. Sep. Sci. Technol. 1985, 19, 857–894. [Google Scholar] [CrossRef]








| Ligand | pKa | Ref. |
|---|---|---|
| EDAB-acac | 10.67 | [This work] |
| pentane-2-dione (acac) | 8.79 | [48] |
| 3-butyl-acetylacetone | 9.60 | [40] |
| 3-allyl- acetylacetone | 9.58 | [40] |
| Ligand. | Metal ion | log β1 | log β2 | log P1 | log P2 | Ref. |
|---|---|---|---|---|---|---|
| acetylacetone | Cu(II) | 8.01 | 9.20 | 3.15 | 5.40 | [40] |
| Co(II) | 5.40 | 9.57 | [48] | |||
| Ni(II) | 5.96 | 10.54 | [48] | |||
| Cu(II) | 8.25 | 15.05 | [48] | |||
| Zn(II) | 5.03 | 8.80 | [48] | |||
| Cd(II) | 3.83 | 6.60 | [48] | |||
| 3-butyl-acetylacetonec | Cu(II) | 7.95 | 9.45 | 2.78 | 6.19 | [40] |
| 3-allyl-acetylacetone | Cu(II) | 8.32 | 10.26 | 2.96 | 7.38 | [40] |
| EDAB-acac | Cu(II) | 4.98 | 10.61 | 3.08 | 6.49 | [this work] |
| Co(II) | 5.26 | 12.73 | 4.03 | 6.82 | [this work] | |
| Ni(II) | 2.15 | 8.85 | 1.44 | 4.71 | [this work] | |
| Zn(II) | 8.29 | 16.84 | 5.76 | 8.67 | [this work] | |
| Cd(II) | 5.15 | 14.50 | 4.95 | 7.12 | [this work] |
| Solutions | Metal Ions | Initial Flux, Ji µmol/m2∙s | Selectivity Order and Selectivity Coefficients |
|---|---|---|---|
| I | Zn(II) | 10.98 | - |
| II | Zn(II) Cd(II) Co(II) | 10.53 7.48 5.39 | Zn(II) > Cd(II) > Co(II) 1.4 2.0 |
| III | Zn(II) Cu(II) Ni(II) | 10.42 6.37 0.50 | Zn(II) > Cu(II) >Ni(II) 1.6 21.0 |
| IV | Zn(II) Co(II) Cu(II) | 9.87 5.61 3.90 | Zn(II) > Co(II) > Cu(II) 1.8 2.5 |
| V | Zn(II) Co(II) Ni(II) | 10.17 5.53 0.40 | Zn(II) > Co(II) > Ni(II) 1.8 25.0 |
| VI | Zn(II) Cd(II) Co(II) Ni(II) | 9.85 7.84 5.72 0.38 | Zn(II) > Cd(II) > Co(II) > Ni(II) 1.3 1.7 26.0 |
| VII | Zn(II) Co(II) Cu(II) Ni(II) | 9.94 5.89 3.61 0.40 | Zn(II) > Co(II) > Cu(II) > Ni(II) 1.7 2.8 25.0 |
| VIII | Zn(II) Cd(II) Co(II) Cu(II) Ni(II) | 9.87 7.43 5.26 3.12 0.20 | Zn(II) > Cd(II) > Co(II) > Cu(II) > Ni(II) 1.3 1.8 3.2 49.0 |
| Mixture | RF, % | |||||
|---|---|---|---|---|---|---|
| Zn | Cd | Co | Cu | Ni | ||
| I | Zn | 99 | ||||
| II | Zn-Cd-Co | 97 | 82 | 71 | ||
| III | Zn-Cu-Ni | 98 | 64 | 15 | ||
| IV | Zn-Co-Cu | 97 | 79 | 61 | ||
| V | Zn-Co-Ni | 97 | 76 | 11 | ||
| VI | Zn-Cd-Co-Ni | 93 | 83 | 75 | 10 | |
| VII | Zn-Co-Cu-Ni | 95 | 73 | 66 | 9 | |
| VIII | Zn-Cd-Co-Cu-Ni | 90 | 76 | 64 | 51 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyszka, I.; Radzyminska-Lenarcik, E. New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes 2020, 10, 385. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10120385
Pyszka I, Radzyminska-Lenarcik E. New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions. Membranes. 2020; 10(12):385. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10120385
Chicago/Turabian StylePyszka, Ilona, and Elzbieta Radzyminska-Lenarcik. 2020. "New Polymer Inclusion Membrane in the Separation of Nonferrous Metal Ion from Aqueous Solutions" Membranes 10, no. 12: 385. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes10120385