Coal Fly Ash Derived Silica Nanomaterial for MMMs—Application in CO2/CH4 Separation
Abstract
:1. Introduction
2. Materials and Methods
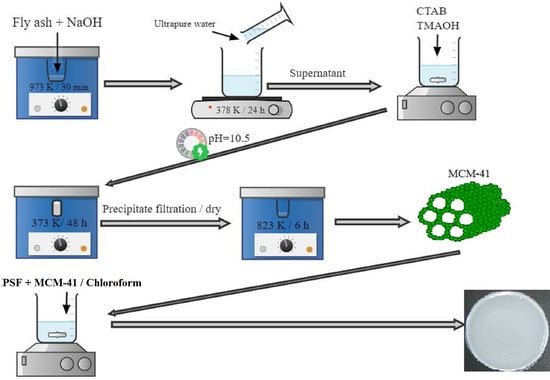
2.1. Obtaining Mesoporous MCM-41
2.2. Obtaining MMMs
2.3. Characterization Techniques
2.4. Gas Permeability Tests
3. Results and Discussion
3.1. Mesoporous MCM-41 Characterization
3.2. Membrane Characterization
3.3. Membrane Testing/Gas Permeation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burmann, P.; Zornoza, B.; Tellez, C.; Coronas, J. MMMs comprising MOFs and porous silicate fillers prepared via spin coating for gas separation. Chem. Eng. Sci. 2014, 107, 66–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas. Con. 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Fernández-Barquín, A.; Casado-Costerillor, C.; Palomino, M.; Valencia, S.; Irabien, A. Permselectivity improvement in membranes for CO2/N2 separation. Sep. Purif. Technol. 2016, 157, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Weng, T.H.; Tseng, H.H.; Wey, M.Y. Fabrication and characterization of poly(phenylene oxide)/SBA-15/carbon molecule sieve multilayer mixed matrix membrane for gas separation. Int. J. Hydrogen Energy 2010, 35, 6971–6983. [Google Scholar] [CrossRef]
- Doong, S.J. Membranes, adsorbent materials and solvent—Based materials for syngas and hydrogen separation. Funct. Mater. Sustain. Energy Appl. 2012, 7, 179–216. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.H. Harnessing filler materials for enhancing biogas separation membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowics, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid crystal template mechanism. Lett. Nat. 1992, 369, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartulli, J.C.; Roth, W.J.; Leonowics, M.E.; Kresge, C.T.; Schmidt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.F.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Yang, H.; Meng, Y.; Yu, C.; Tu, B.; Zhao, D. Understanding effect of wall structure on the hydrothermal stability of mesostructured silica SBA-15. J. Phys. Chem. B 2005, 109, 8723–8732. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak-Kuceba, I.; Nowak, W. Characterization of MCM-41 mesoporous materials derived from polish fly ashes. Int. J. Miner. Process. 2011, 101, 100–111. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, Y.; Yan, J.; Sun, J.; Li, W.; Lu, P. Enhanced CO2 sorption capacity of amine-tethered fly ash residues derived from co-firing of coal and biomass blends. Appl. Energy 2019, 242, 453–461. [Google Scholar] [CrossRef]
- Mazzella, A.; Errico, M.; Spiga, D. CO2 uptake capacity of coal fly ash: Influence of pressure and temperature on direct gas-solid carbonation. J. Environ. Chem. Eng. 2016, 4, 4120–4128. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, C.; Chen, X.; Li, C. CO2 capture and sorbent regeneration performances of some wood ash materials. Appl. Energy 2015, 137, 26–36. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Sun, Q.; Xu, W.; Han, Y. Adsorption of lead ion on amino-functionalized fly-ash-based SBA-15 mesoporous molecular sieves prepared via two-step hydrothermal method. Micropor. Mesopor. Mat. 2017, 252, 105–115. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, Y.; Li, L.; Xu, H.; Sun, Y.; Wang, Y. Novel synthesis of cyano-functionalized mesoporous silica nanospheres (MSN) from coal fly ash for removal of toxic metals from wastewater. J. Hazard. Mater. 2018, 345, 76–86. [Google Scholar] [CrossRef]
- Asl, S.M.H.; Javadian, H.; Khavarpour, M.; Belviso, C.; Taghavi, M.; Maghsudi, M. Porous adsorbents derived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J. Clean. Prod. 2019, 208, 1131–1147. [Google Scholar]
- Niculescu, V.-C.; Miricioiu, M.; Enache, S.; Constantinescu, M.; Bucura, F.; David, E. Optimized method for producing mesoporous silica from incineration ash. Prog. Cryog. Isot. Sep. 2019, 22, 65–76. [Google Scholar]
- Miricioiu, M.G.; Iacob, C.; Nechifor, G.; Niculescu, V.C. High Selective Mixed Membranes Based on Mesoporous MCM-41 and MCM-41-NH2 Particles in a Polysulfone Matrix. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niculescu, V.; Ene, R.; Iordache, I.; Parvulescu, V. Hybrid materials obtained by nickel immobilization on mesoporous MCM-41 and their application in alcohols oxidation. Prog. Cryog. Isot. Sep. 2012, 15, 97–104. [Google Scholar]
- Salam, M.S.A.; Betiha, M.A.; Shaban, S.A.; Elsabagh, A.M.; Abdel-Aal, R.M.; El kady, F.Y. Synthesis and characterization of MCM-41-supported nano zirconia catalysts. Egypt. J. Pet. 2015, 24, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Hoang, V.D.; Dang, T.P.; Dinh, Q.K.; Nguyen, H.P.; Vu, A.T. The synthesis of novel hybrid thiol-functionalized nano-structured SBA-15. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 035011. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Xu, Y.; Sun, G.; Wang, L.; Sun, Y.; Qin, X. Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+ and Cd2+. Colloid. Surface A Physicochem. Eng. Asp. 2009, 349, 61–68. [Google Scholar] [CrossRef]
- Yoshino, H.; Kamiya, K.; Nasu, H. IR Study on the Structural Evolution of Sol-Gel Derived SiO2 Gels in the Early Stage of Conversion to Glasses. J. Non-Cryst. Solids 1990, 126, 68–78. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Ukoba, K.O.; Jen, T.-C. Green technology extraction and characterisation of silica nanoparticles from palm kernel shell ash via sol–gel. J. Mater. Res. Technol. 2020, 9, 307–313. [Google Scholar] [CrossRef]
- Imoisili, P.E.; Ukoba, K.O.; Jen, T.-C. Synthesis and characterization of amorphousmesoporous silica from palm kernel shell ash. Bol. Soc. Esp. Ceram. V. 2020, 59, 159–164. [Google Scholar] [CrossRef]
- Khan, A.L.; Klaysom, C.; Gahlaut, A.; Li, X.; Vankelecom, I.F.J. SPEEK and functionalized mesoporous MCM-41 MMMs for CO2 separations. J. Mater. Chem. 2012, 22, 20057–20064. [Google Scholar] [CrossRef]
- Jomekian, A.; Shafiee, A.; Moradian, A. Synthesis of new modified MCM-41/PSF nanocomposite membrane for improvement of water permeation flux. Desalination Water Treat. 2012, 41, 53–61. [Google Scholar] [CrossRef]
- Razvan, A.; Popa, D.F.; Oprea, O.; Vasile, E.; Dumitru, F.; Nechifor, G. Ultrafiltration MMMs based on mesoporous silica (MCM-41, HMS) embedded in polysulfone. Rev. Chim. 2019, 70, 3089–3093. [Google Scholar] [CrossRef]
- Zornoza, B.; Irusta, S.; Tellez, C.; Coronas, J. Mesoporous silica sphere-polysulfone MMMs for gas separation. Langmuir 2009, 25, 5903–5909. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chung, T.S.; Kulprathipanja, S. Novel Ag+-Zeolite/Polymer MMMs with a high CO2/CH4 selectivity. AIChE J. 2007, 53, 610–616. [Google Scholar] [CrossRef]
- Bos, A.; Punt, I.G.M.; Wessling, M.; Strathmann, H. CO2-induced plasticization phenomena in glassy polymers. J. Membr. Sci. 1999, 155, 67–78. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lorna, W. Penetrant-induced plasticization phenomenon in glassy polymers for gas separation membrane. Sep. Purif. Technol. 2002, 27, 173–194. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Mann Ch’ng, C.W.; Jusoh, N. Tailoring CO2/CH4 Separation Performance of MMMs by Using ZIF-8 Particles Functionalized with Different Amine Groups. Polymers 2019, 11, 2042. [Google Scholar] [CrossRef] [Green Version]
- Ozen, H.A.; Ozturk, B. Gas separation characteristic of mixed matrix membrane prepared by MOF-5 including different metals. Sep. Purif. Technol. 2019, 211, 514–521. [Google Scholar] [CrossRef]
- Reid, B.D.; Ruiz-Trevino, F.A.; Musselman, I.H.; Balkus, K.J.; Ferraris, J.P. Gas permeability properties of polysulfone membranes containing the mesoporous molecular sieve MCM-41. Chem. Mater. 2001, 13, 2366–2373. [Google Scholar] [CrossRef]
- Kim, S.; Marand, E. High permeability nano-composite membranes based on mesoporous MCM-41 nanoparticles in a polysulfone matrix, Microporous and Mesoporous. Materials 2008, 114, 129–136. [Google Scholar]
- Murali, R.S.; Ismail, A.F.; Rahman, M.A.; Sridhar, S. MMMs of Pebax-1657 loaded with 4A zeolite for gaseous separations. Sep. Purif. Technol. 2014, 129, 1–8. [Google Scholar] [CrossRef]
















| Element | Fly Ash (%wt.) * | Est. Error | MCM-41 (%wt.) ** | Est. Error |
|---|---|---|---|---|
| Si | 23.4300 | 0.1200 | 45.5400 | 0.0400 |
| Al | 14.6500 | 0.1200 | 0.0670 | 0.0054 |
| Fe | 6.7200 | 0.1000 | 0.0178 | 0.0009 |
| K | 3.1100 | 0.0800 | 0.0176 | 0.0010 |
| Ca | 2.6200 | 0.0700 | 0.0077 | 0.0006 |
| Mg | 1.0800 | 0.0400 | - | - |
| Ti | 0.7390 | 0.0300 | - | - |
| S | 0.3500 | 0.0170 | - | - |
| Na | 0.4210 | 0.0310 | 0.6300 | 0.0310 |
| P | 0.0953 | 0.0048 | - | |
| Ba | 0.0883 | 0.0044 | - | |
| Mn | 0.0551 | 0.0028 | - | |
| V | 0.0276 | 0.0014 | - | |
| Sr | 0.0409 | 0.0020 | - | |
| Cr | 0.0268 | 0.0013 | - | |
| Zr | 0.0211 | 0.0011 | - | |
| Ni | 0.0195 | 0.0010 | - | |
| Zn | 0.0194 | 0.0010 | - | |
| Rb | 0.0178 | 0.0009 | - | |
| Cu | 0.0131 | 0.0007 | - | |
| Ce | 0.0116 | 0.0024 | - |
| Silica Loading wt.% | Permeability, Barrer | Ideal Selectivity (PCO2/PCH4) | Real Selectivity (CO2/CH4–30/70%) | |
|---|---|---|---|---|
| CO2 | CH4 | |||
| 0 | 3.58 ± 0.18 | 0.16 ± 0.01 | 22.4 | 20.3 |
| 15 | 7.23 ± 0.37 | 0.33 ± 0.02 | 21.9 | 18.9 |
| 25 | 8.67 ± 0.43 | 0.41 ± 0.02 | 21.1 | 18.2 |
| 35 | 14.52 ± 0.73 | 0.83 ± 0.04 | 17.5 | 15.3 |
| Mixed Matrix Membrane | Permeability (Barrer) | Ideal Selectivity (PCO2/PCH4) | Testing Condition | Reference | |
|---|---|---|---|---|---|
| CO2 | CH4 | ||||
| 5% MOF-5/PI | ~3 | ~0.4 | ~7.5 | 1 Bar, 298 K | [38] |
| 5% CuMOF-5/PI | ~4 | ~0.5 | ~8.0 | 1 Bar, 298 K | [38] |
| 5% CuCoMOF-5/PI | ~4.2 | ~0.5 | ~8.4 | 1 Bar, 298 K | [38] |
| 10% MCM-41/PSF | 10.5 | 0.6 | 17.5 | 2 Bar, 298 K | [39] |
| 20% MCM-41/PSF | 11.4 | 0.6 | 19.0 | 2 Bar, 298 K | [39] |
| 30% MCM-41/PSF | 20.5 | 1.0 | 20.5 | 2 Bar, 298 K | [39] |
| 10% MCM-41/PSF | 6.6 | 0.3 | 22.0 | 4.04 Bar, 308 K | [40] |
| 20% MCM-41/PSF | 7.8 | 0.3 | 26.0 | 4.04 Bar, 308 K | [40] |
| 40% MCM-41/PSF | 14.8 | 1.0 | 14.8 | 4.04 Bar, 308 K | [40] |
| 5% 4A Zeolite/Pebax-1657 | 71.4 | 2.2 | 32.5 | 4.9 Bar, 308 K | [41] |
| 10% 4A Zeolite/Pebax-1657 | 97.0 | 3.7 | 26.2 | 4.9 Bar, 308 K | [41] |
| 20% 4A Zeolite/Pebax-1657 | 113.7 | 6.5 | 17.5 | 4.9 Bar, 308 K | [41] |
| 30% 4A Zeolite/Pebax-1657 | 155.8 | 19.7 | 7.9 | 4.9 Bar, 308 K | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miricioiu, M.G.; Niculescu, V.-C.; Filote, C.; Raboaca, M.S.; Nechifor, G. Coal Fly Ash Derived Silica Nanomaterial for MMMs—Application in CO2/CH4 Separation. Membranes 2021, 11, 78. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11020078
Miricioiu MG, Niculescu V-C, Filote C, Raboaca MS, Nechifor G. Coal Fly Ash Derived Silica Nanomaterial for MMMs—Application in CO2/CH4 Separation. Membranes. 2021; 11(2):78. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11020078
Chicago/Turabian StyleMiricioiu, Marius Gheorghe, Violeta-Carolina Niculescu, Constantin Filote, Maria Simona Raboaca, and Gheorghe Nechifor. 2021. "Coal Fly Ash Derived Silica Nanomaterial for MMMs—Application in CO2/CH4 Separation" Membranes 11, no. 2: 78. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes11020078