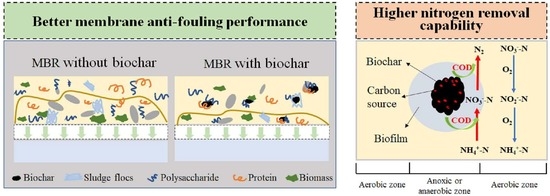
Biochar Addition in Membrane Bioreactor Enables Membrane Fouling Alleviation and Nitrogen Removal Improvement for Low C/N Municipal Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Rig Set-Up and Operating Conditions
2.2. Biochar Characteristics
2.3. Biochar Carbon Source Release Experiment
2.4. Membrane Fouling Analysis
2.5. Microbial Community Analyses
2.6. Analytical Methods
3. Results and Discussion
3.1. Impact of Biochar Addition on Membrane Bioreactor Treatment Performance
3.2. Impact of Biochar Addition on Mixed Liquor Suspensions
3.3. Impact of Biochar Addition on Membrane Fouling Propensity and Fouling Mechanism
3.4. Impact of Biochar Addition on Microbial Diversity
4. Conclusions
- Biochar addition can enable membrane fouling alleviation and nitrogen removal improvement.
- Biochar addition can effectively alleviate membrane fouling because of the change of mixed liquor, such as lower SMP content reduction by about 14%, lower SMP P/C ratio from 0.28 ± 0.02 to 0.22 ± 0.03, smaller SMP molecular weight and bigger particle size from 67.68 ± 6.9 μm to 113.47 ± 4.8 μm. This can be mainly ascribed to the biochar adsorption capacity and facilitate the formation of bigger flocs with the carbon skeleton in biochar as a core.
- The improved nitrogen removal after biochar addition can be due to the initial carbon source release from biochar and formation of aerobic–anaerobic microstructures.
- Microbial diversity analysis results suggested more accumulation of denitrification microbes, including norank_f__JG30-KF-CM45 and Plasticicumulans. A less relative abundance of Aeromonas after biochar addition suggested less EPS secretion, thus reducing the rate of membrane fouling.
- Further studies about biochar modification and carbon source release kinetics should be conducted in order to increase the carbon source content, control the release rate and fit the carbon source demand from the denitrification bacteria. Additionally, the cost analyses of biochar addition on long-term performance should be carried out.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, L.; Guo, W.; Ngo, H.H.; Zhang, X.; Chen, C.; Chen, Z.; Cheng, D.; Ni, S.; Wang, Q. Recent advances in attached growth membrane bioreactor systems for wastewater treatment. Sci. Total Environ. 2022, 808, 152123. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, S.; Wang, X.; Chen, C.; Huang, X. Current state and challenges of full-scale membrane bioreactor applications: A critical review. Bioresour. Technol. 2019, 271, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Mirbagheri, S.A. Critical review of fouling mitigation strategies in membrane bioreactors treating water and wastewater. Bioresour. Technol. 2018, 258, 318–334. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.; Drews, A.; Kraume, M.; Shin, H.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Charfi, A.; Lesage, G.; Heran, M.; Kim, J. Membrane bioreactors for wastewater treatment: A review of mechanical cleaning by scouring agents to control membrane fouling. Chem. Eng. J. 2017, 307, 897–913. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Miao, J.; Li, Y. A novel approach for mitigation of membrane fouling: Concomitant use of flocculant and magnetic powder. Bioresour. Technol. 2016, 209, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Remy, M.; Potier, V.; Temmink, H.; Rulkens, W. Why low powdered activated carbon addition reduces membrane fouling in MBRs. Water Res. 2010, 44, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Yang, S.; Li, X.; Wen, W.; Huang, X.; Yang, Y.; Wang, X.; Li, Y.; Sano, D.; Chen, R. Revisiting the effects of powdered activated carbon on membrane fouling mitigation in an anaerobic membrane bioreactor by evaluating long-term impacts on the surface layer. Water Res. 2019, 167, 115137. [Google Scholar] [CrossRef]
- Sohn, W.; Guo, W.; Ngo, H.H.; Deng, L.; Cheng, D. Powdered activated carbon addition for fouling control in anaerobic membrane bioreactor. Bioresour.Technol. Rep. 2021, 15, 100721. [Google Scholar] [CrossRef]
- Aslam, M.; Yang, P.; Lee, P.H.; Kim, J. Novel staged anaerobic fluidized bed ceramic membrane bioreactor: Energy reduction, fouling control and microbial characterization—ScienceDirect. J. Membr. Sci. 2018, 553, 200–208. [Google Scholar] [CrossRef]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Sima, X.F.; Wang, Y.Y.; Shen, X.C.; Jing, X.R.; Jiang, H. Robust bio-charassisted alleviation of membrane fouling in MBRs by indirect mechanism. Sep. Purif. Technol. 2017, 184, 195–204. [Google Scholar] [CrossRef]
- Chang, M.; Liang, B.; Zhang, K.; Wang, Y.; Jin, D.; Zhang, Q.; Hao, L.; Zhu, T. Simultaneous shortcut nitrification and denitrification in a hybrid membrane aerated biofilms reactor (H-MBfR) for nitrogen removal from low COD/N wastewater. Water Res. 2022, 211, 118027. [Google Scholar] [CrossRef] [PubMed]
- Pynaert, K.; Smets, B.F.; Beheydt, D.; Verstraete, W. Start-up of autotrophic nitrogen removal reactors via sequential biocatalyst addition. Environ. Sci. Technol. 2004, 38, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Dhamole, P.B.; Nair, R.R.; D’Souza, S.F.; Lele, S.S. Simultaneous removal of carbon and nitrate in an airlift bioreactor. Bioresour. Technol. 2009, 100, 1082–1086. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, J.; Ren, Z.; Guo, G.; Wang, C.; Wang, H. Effect of COD/N and COD/P ratios on the PHA transformation and dynamics of microbial community structure in a denitrifying phosphorus removal process: Nutrient ratios effect on PHA transformation in a A 2 N-SBR. J. Chem. Technol. Biotechnol. 2013, 88, 1228–1236. [Google Scholar] [CrossRef]
- Han, F.; Ye, W.; Wei, D.; Xu, W.; Du, B.; Wei, Q. Simultaneous nitrification-denitrification and membrane fouling alleviation in a submerged biofilm membrane bioreactor with coupling of sponge and biodegradable PBS carrier. Bioresour. Technol. 2018, 270, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liss, S.N.; Liao, B.Q. Influence of COD:N ratio on sludge properties and their role in membrane fouling of a submerged membrane bioreactor. Water Res. 2016, 89, 132–141. [Google Scholar] [CrossRef]
- Sima, X.F.; Li, B.B.; Jiang, H. Influence of pyrolytic biochar on settleability and denitrification of activated sludge process. Chin. J. Chem. Phys. 2017, 30, 357–364. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Wang, D.; Jin, Y. Effects of bamboo charcoal on fouling and microbial diversity in a flat-sheet ceramic membrane bioreactor. Bioresour. Technol. 2017, 243, 1020–1026. [Google Scholar] [CrossRef]
- Jamal Khan, S.; Visvanathan, C.; Jegatheesan, V. Effect of powdered activated carbon (PAC) and cationic polymer on biofouling mitigation in hybrid MBRs. Bioresour. Technol. 2012, 113, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ahn, W.; Lee, C. Comparison of the filtration characteristics between attached and suspended growth microorganisms in submerged membrane bioreactor. Water Res. 2001, 35, 2435–2445. [Google Scholar] [CrossRef]
- Wang, K.M.; Zhang, L.J.; Zhang, H.L.; Li, J.L.; Zhang, Y.C.; Liu, B.C.; Wang, H.Y. Membrane fouling amelioration through pseudo dead-end filtration coupled with transmembrane pressure (TMP) set-point control in an anaerobic membrane bioreactor for municipal wastewater treatment. Environ. Sci.-Water Res. Technol. 2021, 7, 221–232. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Wang, K.M.; Jiang, S.F.; Zhang, Z.H.; Ye, Q.Q.; Zhang, Y.C.; Zhou, J.H.; Hong, Q.K.; Yu, J.M.; Wang, H.Y. Impact of static biocarriers on the microbial community, nitrogen removal and membrane fouling in submerged membrane bioreactor at different COD:N ratios. Bioresour. Technol. 2020, 301, 122798. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.; Rosenburgh, N.; Farr, A. Protein measurements with the folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1951, 28, 350–356. [Google Scholar] [CrossRef]
- Mao, X.; Myavagh, P.H.; Lotfikatouli, S.; Hsiao, B.S.; Walker, H.W. Membrane bioreactors for nitrogen removal from wastewater: A review. J. Environ. Eng. 2020, 146, 03120002. [Google Scholar] [CrossRef]
- Sarioglu, M.; Insel, G.; Artan, N.; Orhon, D. Model evaluation of simultaneous nitrification and denitrification in a membrane bioreactor operated without an anoxic reactor. J. Membr. Sci. 2009, 337, 17–27. [Google Scholar] [CrossRef]
- Foereid, B. Biochar in nutrient recycling—The effect and its use in wastewater treatment. Open J. Soil. Sci. 2015, 5, 6. [Google Scholar] [CrossRef]
- Fu, X.; Hou, R.; Yang, P.; Qian, S.; Feng, Z.; Chen, Z.; Wang, F.; Yuan, R.; Chen, H.; Zhou, B. Application of external carbon source in heterotrophic denitrification of domestic sewage: A review. Sci. Total. Environ. 2022, 817, 153061. [Google Scholar] [CrossRef]
- He, X.; Zhang, S.; Jiang, Y.; Li, M.; Yuan, J.; Wang, G. Influence mechanism of filling ratio on solid-phase denitrification with polycaprolactone as biofilm carrier. Bioresour. Technol. 2021, 337, 125401. [Google Scholar] [CrossRef]
- Yu, L.; Cheng, L.; Peng, Z.; Li, T.; Liu, L.; Liu, X.; Fan, P. Carbon release mechanism of synthetic and agricultural solid carbon sources. Water Environ. J. 2020, 34, 121–130. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Yan, G.; Dong, W.; Chu, Z.; Wang, H.; Chang, Y.; Ling, Y.; Zhang, Y. Initial carbon release characteristics, mechanisms and denitrification performance of a novel slow release carbon source. J. Environ. Sci. 2022, 118, 32–45. [Google Scholar] [CrossRef]
- Weng, Z.; Ma, H.; Ma, J.; Kong, Z.; Shao, Z.; Yuan, Y.; Xu, Y.; Ni, Q.; Chai, H. Corncob-pyrite bioretention system for enhanced dissolved nutrient treatment: Carbon source release and mixotrophic denitrification. Chemosphere 2022, 306, 135534. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Guo, W.; Ngo, H.H.; Zhang, H.; Wang, J.; Li, J.; Xia, S.; Wu, Y. Biofouling and control approaches in membrane bioreactors. Bioresour. Technol. 2016, 221, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.; Chae, S. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Ye, L.; Xia, T.; Chen, H.; Ling, L.; Xu, X.; Alvarez, P.J.J.; Zhu, L. Effect of bamboo charcoal amendment on an AnMBR in the aspect of anaerobic habitat and membrane fouling. Environ. Sci. Water Res. Technol. 2018, 4, 2058–2069. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation−emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- He, Q.; Song, Q.; Zhang, S.; Zhang, W.; Wang, H. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: Reactor performance, extracellular polymeric substances and microbial successions. Chem. Eng. J. 2018, 331, 841–849. [Google Scholar] [CrossRef]
- Jiang, W.; Xia, S.; Liang, J.; Zhang, Z.; Hermanowicz, S.W. Effect of quorum quenching on the reactor performance, biofouling and biomass characteristics in membrane bioreactors. Water Res. 2013, 47, 187–196. [Google Scholar] [CrossRef]
- Hazrati, H.; Jahanbakhshi, N.; Rostamizadeh, M. Fouling reduction in the membrane bioreactor using synthesized zeolite nano-adsorbents. J. Membr. Sci. 2018, 555, 455–462. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, J.; Zuo, X.; Liao, W.; Ma, C.; He, J.; Chen, Z. Characteristics of the sludge filterability and microbial composition in PAC hybrid MBR: Effect of PAC replenishment ratio. Biochem. Eng. J. 2019, 145, 10–17. [Google Scholar] [CrossRef]
- Zhang, M.; Hong, H.; Lin, H.; Shen, L.; Yu, H.; Ma, G.; Chen, J.; Liao, B.-Q. Mechanistic insights into alginate fouling caused by calcium ions based on terahertz time-domain spectra analyses and DFT calculations. Water Res. 2018, 129, 337–346. [Google Scholar] [CrossRef]
- Salanitro, J.P.; Johnson, P.C.; Spinnler, G.E.; Maner, P.M.; Wisniewski, H.L.; Bruce, C. Field-scale demonstration of enhanced MTBE bioremediation through aquifer bioaugmentation and oxygenation. Environ. Sci. Technol. 2000, 34, 4152–4162. [Google Scholar] [CrossRef]
- Yang, X.; Song, H.; Lu, J.; Fu, D.; Cheng, B. Influence of diatomite addition on membrane fouling and performance in a submerged membrane bioreactor. Bioresour. Technol. 2010, 101, 9178–9184. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane fouling caused by biological foams in a submerged membrane bioreactor: Mechanism insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef] [PubMed]
- Alizad Oghyanous, F.; Etemadi, H.; Yegani, R. Foaming control and determination of biokinetic coefficients in membrane bioreactor system under various organic loading rate and sludge retention time. Biochem. Eng. J. 2020, 157, 107491. [Google Scholar] [CrossRef]
- Bezirgiannidis, A.; Marinakis, N.; Ntougias, S.; Melidis, P. Membrane bioreactor performance during processing of a low carbon to nitrogen ratio municipal wastewater. Environ. Process 2018, 5, 87–100. [Google Scholar] [CrossRef]
- Palmarin, M.J.; Young, S. The effects of biocarriers on the mixed liquor characteristics, extracellular polymeric substances, and fouling rates of a hybrid membrane bioreactor. Biochem. Eng. J. 2019, 141, 278–284. [Google Scholar] [CrossRef]
- Gharibian, S.; Hazrati, H. Towards practical integration of MBR with electrochemical AOP: Improved biodegradability of real pharmaceutical wastewater and fouling mitigation. Water Res. 2022, 218, 118478. [Google Scholar] [CrossRef]
- Kumar, M.; Adham, S.S.; Pearce, W.R. Investigation of seawater reverse osmosis fouling and its relationship to pretreatment type. Environ. Sci. Technol. 2006, 40, 2037–2044. [Google Scholar] [CrossRef]
- Kim, I.S.; Jang, N. The effect of calcium on the membrane biofouling in the membrane bioreactor (MBR). Water Res. 2006, 40, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Croué, J.P.; Benedetti, M.F.; Violleau, D.; Leenheer, J.A. Characterization and copper binding of humic and nonhumic organic matter isolated from the south platte river: Evidence for the presence of nitrogenous binding site. Environ. Sci. Technol. 2003, 37, 328–336. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Wang, L.; He, J.; Li, B.; Zhang, Y.; Xing, X.H. Effects of furan derivatives on biohydrogen fermentation from wet steam-exploded cornstalk and its microbial community. Bioresour. Technol. 2015, 175, 152–159. [Google Scholar] [CrossRef]
- Huang, W.; She, Z.; Gao, M.; Wang, Q.; Jin, C.; Zhao, Y.; Guo, L. Effect of anaerobic/aerobic duration on nitrogen removal and microbial community in a simultaneous partial nitrification and denitrification system under low salinity. Sci. Total Environ. 2019, 651, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, Z.; Ma, J.; Zhu, C.; Li, Y.; Wu, Z. Membrane bioreactors fed with different COD/N ratio wastewater: Impacts on microbial community, microbial products, and membrane fouling. Environ. Sci. Pollut. Res. 2015, 22, 11436–11445. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Li, J.; Ngo, H.H.; Guo, W.; Hu, J.; Gao, M.; Wang, Q.; Hou, Y. Effect of magnetic powder on membrane fouling mitigation and microbial community/composition in membrane bioreactors (MBRs) for municipal wastewater treatment. Bioresour. Technol. 2018, 249, 377–385. [Google Scholar] [CrossRef]
- Lu, H.; Chandran, K.; Stensel, D. Microbial ecology of denitrification in biological wastewater treatment. Water Res. 2014, 64, 237–254. [Google Scholar] [CrossRef]
- Ouyang, E.; Liu, Y.; Ouyang, J.; Wang, X. Effects of different wastewater characteristics and treatment techniques on the bacterial community structure in three pharmaceutical wastewater treatment systems. Environ. Technol. 2019, 40, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M.; Cırık, K.; Dolaz, M.; Başak, S. Evaluation of textile wastewater treatment in sequential anaerobic moving bed bioreactor—aerobic membrane bioreactor. Process Biochem. 2021, 105, 62–71. [Google Scholar] [CrossRef]
- Huang, X.; Xing, Y.X.; Wang, H.J.; Dai, Z.Y.; Chen, T.T. Nitrogen advanced treatment of urban sewage by denitrification deep-bed filter: Removal performance and metabolic pathway. Front. Microbiol. 2022, 12, 811697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, K.T.; Jiang, W.L.; He, J.Y.; Wang, H.; Li, B.; Gao, M. Black odorous water concentrating by forward osmosis (FO) with aquaporin biomimetic membranes: Pollutants concentrating and membrane fouling characteristics. Chem. Eng. J. 2020, 400, 125938. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, R.X.; Song, J.; Ren, Y.; Luo, X.; Li, Y.; Li, X.; Li, T.; Wang, X.; Zhou, Q. Combined phyto-microbial-electrochemical system enhanced the removal of petroleum hydrocarbons from soil: A profundity remediation strategy. J. Hazard. Mater 2021, 420, 126592. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zeng, Q.; Hou, Y.; Li, H.; Liu, J.; Xu, J.; Shi, S.; Ma, F. Impacts of long-term electric field applied on the membrane fouling mitigation and shifts of microbial communities in EMBR for treating phenol wastewater. Sci. Total Environ. 2020, 716, 137139. [Google Scholar] [CrossRef] [PubMed]






| Parameter | CMBR | BMBR | ||
|---|---|---|---|---|
| Effluent Characteristics mg L−1 | Removal % | Effluent Characteristics mg L−1 | Removal % | |
| COD | 9.8 ± 0.9 | 96.4 ± 0.3 | 5.5 ± 0.9 | 97.9 ± 0.3 |
| NH4+-N | 0.8 ± 0.05 | 98.2 ± 0.1 | 0.7 ± 0.05 | 98.4 ± 0.1 |
| NO3−-N | 21.1 ± 1.5 | - | 16.7 ± 1.2 * | - |
| TN | 21.9 ± 1.5 | 62.3 ± 0.5 | 17.4 ± 1.2 * | 70.1 ± 0.6 * |
| Parameter | SVI (mL/g) | SV30 (%) | Particle Size (μm) | Zeta Potential (−mV) |
|---|---|---|---|---|
| CMBR | 128.1 ± 6 | 98.0 ± 0.8 | 67.68 ± 6.9 | 20.6 ± 0.44 |
| BMBR | 90.5 ± 7 | 87.0 ± 0.8 | 113.47 ± 4.8 | 19.4 ± 0.30 |
| Rt | Rm | Rp | Rc | |
|---|---|---|---|---|
| CMBR (×1012 m−1) | 6.68 | 1.45 | 0.41 | 4.82 |
| Percent of Rt | 100% | 21.74% | 6.10% | 72.16% |
| BMBR (×1012 m−1) | 6.70 | 1.45 | 0.079 | 5.17 |
| Percentage of Rt | 100% | 21.66% | 1.18% | 77.16% |
| Sample/Estimators | Shannon | Simpson | Ace | Chao | Pd | OTU |
|---|---|---|---|---|---|---|
| CMBR | 4.426 | 0.043 | 863.633 | 863.065 | 84.134 | 776 |
| BMBR | 4.651 | 0.043 | 967.221 | 973.280 | 93.096 | 890 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Ye, Q.; Shen, Y.; Wang, Y.; Hong, Q.; Zhang, C.; Liu, M.; Wang, H. Biochar Addition in Membrane Bioreactor Enables Membrane Fouling Alleviation and Nitrogen Removal Improvement for Low C/N Municipal Wastewater Treatment. Membranes 2023, 13, 194. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes13020194
Wang K, Ye Q, Shen Y, Wang Y, Hong Q, Zhang C, Liu M, Wang H. Biochar Addition in Membrane Bioreactor Enables Membrane Fouling Alleviation and Nitrogen Removal Improvement for Low C/N Municipal Wastewater Treatment. Membranes. 2023; 13(2):194. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes13020194
Chicago/Turabian StyleWang, Kanming, Qiaoqiao Ye, Yuxiang Shen, Yajing Wang, Qiankun Hong, Chenlong Zhang, Min Liu, and Hongyu Wang. 2023. "Biochar Addition in Membrane Bioreactor Enables Membrane Fouling Alleviation and Nitrogen Removal Improvement for Low C/N Municipal Wastewater Treatment" Membranes 13, no. 2: 194. https://0-doi-org.brum.beds.ac.uk/10.3390/membranes13020194