Numerical Study of Epitaxial Growth after Partial Remelting during Selective Electron Beam Melting in the Context of Ni–Al
Abstract
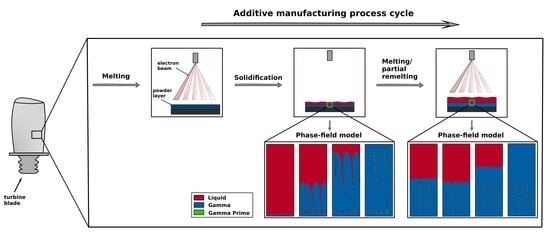
:1. Introduction
2. Materials and Methods
2.1. Model Description
2.2. Applied Input Data
3. Results and Discussion
3.1. Microstructure Formation during Solidification
3.2. Microstructure Evolution during Remelting
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Face-cubic-centred matrix phase in the microstructure of the referenced system | |
| ’ | Intermetallic phase forming during the eutectic reaction at the end of solidification |
| e.g. | for example |
| BC | Boundary condition |
| SEBM | Selective Electron beam melting process |
| AM | Additive Manufacturing |
| avg. | Averaged |
| temp. | Temperature |
| Z-direc. | Z-direction |
| X-direc. | Z-direction |
| c | Al concentration |
| Al. conc. | Al concentration |
| J | Anti-trapping current |
| t | Time step |
| T | Temperature value present time step |
| T | Temperature value previous time step |
| Time increment | |
| Err. | Error |
| DC | Diffusion coefficient |
Appendix A
References
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Körner, C. Additive manufacturing of metallic components by selective electron beam melting—A review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Ramsperger, M.; Singer, R.; Körner, C. Microstructure of the nickel-base superalloy CMSX-4 fabricated by selective electron beam melting. Metall. Mater. Trans. A 2016, 1469–1480. [Google Scholar] [CrossRef] [Green Version]
- Chauvet, E.; Tassin, C.; Blandin, J.J.; Dendievel, R.; Martin, G. Producing Ni-base superalloys single crystal by selective electron beam melting. Scr. Mater. 2018, 152, 15–19. [Google Scholar] [CrossRef]
- Parsa, A.; Ramsperger, M.; Kostka, A.; Somsen, C.; Körner, C.; Eggeler, G. Transmission electron microscopy of a CMSX-4 Ni-base superalloy produced by selective electron beam melting. Metals 2016, 6, 258. [Google Scholar] [CrossRef] [Green Version]
- You, Q.; Yuan, H.; You, X.; Li, J.; Zhao, L.; Shi, S.; Tan, Y. Segregation behavior of nickel-based superalloy after electron beam smelting. Vacuum 2017, 145, 116–122. [Google Scholar] [CrossRef]
- Bürger, D.; Parsa, A.; Ramsperger, M.; Körner, C.; Eggeler, G. Creep properties of single crystal Ni-base superalloys (SX): A comparison between conventionally cast and additive manufactured CMSX-4 materials. Mater. Sci. Eng. A 2019, 762, 138098. [Google Scholar] [CrossRef]
- Nie, P.; Ojo, O.; Li, Z. Numerical modeling of microstructure evolution during laser additive manufacturing of a nickel-based superalloy. Acta Mater. 2014, 77, 85–95. [Google Scholar] [CrossRef]
- Rodgers, T.; Moser, D.; Abdeljawad, F.; Jackson, O.U.; Carroll, J.; Jared, B.; Bolintineanu, D.; Mitchell, J.; Madison, J. Simulation of powder bed metal additive manufacturing microstructures with coupled finite difference-Monte Carlo method. Addit. Manuf. 2021, 41, 101953. [Google Scholar] [CrossRef]
- Zinovieva, O.; Zinoviev, A.; Ploshikhin, V. Three-dimensional modeling of the microstructure evolution during metal additive manufacturing. Comput. Mater. Sci. 2018, 141, 207–220. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, Z.; Yao, X.; Hu, C.; Ge, P.; Wan, Z.; Li, J.; Wu, Q. Numerical methods for microstructural evolutions in laser additive manufacturing. Comput. Math. Appl. 2019, 78, 2296–2307. [Google Scholar] [CrossRef]
- Steinbach, I. Why solidification? Why phase-field? JOM 2013, 65, 1096–1102. [Google Scholar] [CrossRef] [Green Version]
- Clarke, A.; Tourret, D.; Song, Y.; Imhoff, S.; Gibbs, P.; Gibbs, J.; Fezzaa, K.; Karma, A. Microstructure selection in thin-sample directional solidification of an Al-Cu alloy: In situ X-ray imaging and phase-field simulations. Acta Mater. 2017, 129, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, R. Modeling and numerical simulations of dendritic crystal growth. Phys. D Nonlinear Phenom. 1993, 63, 410–423. [Google Scholar] [CrossRef]
- Warnken, N.; Ma, D.; Drevermann, A.; Reed, R.; Fries, S.; Steinbach, I. Phase-field modelling of as-cast microstructure evolution in nickel-based superalloys. Acta Mater. 2009, 57, 5862–5875. [Google Scholar] [CrossRef]
- Schwerdtfeger, J.; Körner, C. Selective electron beam melting of Ti–48Al–2Nb–2Cr: Microstructure and aluminium loss. Intermetallics 2014, 49, 29–35. [Google Scholar] [CrossRef]
- Koepf, J.; Gotterbarm, M.; Markl, M.; Körner, C. 3D multi-layer grain structure simulation of powder bed fusion additive manufacturing. Acta Mater. 2018, 152, 119–126. [Google Scholar] [CrossRef]
- Steinbach, I.; Pezzolla, F.; Nestler, B.; Seeßelberg, M.; Prieler, R.; Schmitz, G.; Rezende, J. A phase field concept for multiphase systems. Phys. D 1996, 94, 135–147. [Google Scholar] [CrossRef]
- Kundin, J.; Mushongera, L.; Emmerich, H. Phase-field modeling of microstructure formation during rapid solidification in Inconel 718 superalloy. Acta Mater. 2015, 95, 343–356. [Google Scholar] [CrossRef]
- Warnken, N. Studies on the solidification path of single crystal superalloys. J. Phase Equilib. Diffus. 2016, 37, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Takaki, T.; Sakane, S.; Ohno, M.; Shibuta, Y.; Shimokawabe, T.; Aoki, T. Primary arm array during directional solidification of a single-crystal binary alloy: Large-scale phase-field study. Acta Mater. 2016, 118, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, I.; Pezzolla, F. A generalized field method for multiphase transformations using interface fields. Phys. D 1999, 134, 385–393. [Google Scholar] [CrossRef]
- Steinbach, I. Phase-field models in materials science. Model. Simul. Mater. Sci. Eng. 2009, 17, 073001. [Google Scholar] [CrossRef]
- Karma, A. Phase-Field Formulation for Quantitative Modeling of Alloy Solidification. Phys. Rev. Lett. 2001, 87, 115701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S. A phase-field model with antitrapping current for multicomponent alloys with arbitrary thermodynamic properties. Acta Mater. 2007, 55, 4391–4399. [Google Scholar] [CrossRef]
- Echebarria, B.; Folch, R.; Karma, A.; Plapp, M. Quantitative phase-field model of alloy solidification. Phys. Rev. E 2004, 70, 061604. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://openphase-solutions.com (accessed on 20 October 2021).
- Tegeler, M.; Shchyglo, O.; Kamachali, R.D.; Monas, A.; Steinbach, I.; Sutmann, G. Parallel multiphase field simulations with OpenPhase. Comput. Phys. Commun. 2017, 215, 173–187. [Google Scholar] [CrossRef]
- Available online: https://thermocalc.com (accessed on 20 October 2021).
- Greer, A.; Bunn, A.; Tronche, A.; Evans, P.; Bristow, D. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Monas, A.; Shchyglo, O.; Hoeche, D.; Tegeler, M.; Steinbach, I. Dual-scale phase-field simulation of Mg-Al alloy solidification. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Tucson, AZ, USA, 28 June–2 July 2015; Volume 84. [Google Scholar]
- Körner, C.; Ramsperger, M.; Meid, C.; Bürger, D.; Wollgramm, P.; Bartsch, M.; Eggeler, G. Microstructure and mechanical properties of CMSX-4 single crystals prepared by additive manufacturing. Metall. Mater. Trans. A 2018, 49, 3781–3792. [Google Scholar] [CrossRef] [Green Version]
- Karimi, P.; Sadeghi, E.; Åkerfeldt, P.; Ålgårdh, J.; Andersson, J. Influence of successive thermal cycling on microstructure evolution of EBM-manufactured alloy 718 in track-by-track and layer-by-layer design. Mater. Des. 2018, 160, 427–441. [Google Scholar] [CrossRef]
- Klassen, A.; Scharowsky, T.; Körner, C. Evaporation model for beam based additive manufacturing using free surface lattice Boltzmann methods. J. Phys. D Appl. Phys. 2014, 47, 275–303. [Google Scholar] [CrossRef]






| t [s] | |||||
|---|---|---|---|---|---|
| 10 | 1.303110 | 3.41610 | 6.693110 | 1.253110 | 1.099710 |
| 100 | 1.668010 | 1.090510 | 1.598410 | 6.555810 | 4.604610 |
| 1000 | 6.806610 | 1.808610 | 1.80910 | 1.812810 | 1.852510 |
| 10,000 | 7.834410 | 6.654310 | 6.654310 | 6.654110 | 6.652810 |
| Parameter | Value |
|---|---|
| m (slope from Figure 1) | −8.0136 K/mole-% Al |
| m | −8.8347 K/mole-% Al |
| m | 2.9846 K/mole-% Al |
| m | 6.9373 K/mole-% Al |
| m | 77.7003 K/mole-% Al |
| m | 188.5815 K/mole-% Al |
| Domain size (x, y, z) | (2001625) cells |
| Grid spacing | 8.010 m |
| Time increment | 1.010 s |
| Molar Volume Liquid | 7.6410 |
| Molar Volume | 7.2710 |
| Molar Volume ’ | 7.010 |
| Interface energy liquid-, liquid-’ | 0.26 Jm |
| Start temperature | 1663.0 K |
| Cooling rate | −10,000 Ks |
| Latent heat Liq.→ | 139,252.71 JKg |
| Latent heat Liq.’ | 98,396.52 JKg |
| Thermal conductivity liquid | 50.0 WmK |
| Thermal conductivity , ’ | 75.0 WmK |
| Heat capacity liquid | 810.75 JK |
| Heat capacity , ’ | 697.21 JK |
| Interface mobility | 7.510 mJs |
| Interface mobility ’ | 9.010 mJs |
| Entropy of fusion | −0.812410 JmK |
| Entropy of fusion ’ | −1.218810 JmK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaar, H.; Steinbach, I.; Tegeler, M. Numerical Study of Epitaxial Growth after Partial Remelting during Selective Electron Beam Melting in the Context of Ni–Al. Metals 2021, 11, 2012. https://0-doi-org.brum.beds.ac.uk/10.3390/met11122012
Schaar H, Steinbach I, Tegeler M. Numerical Study of Epitaxial Growth after Partial Remelting during Selective Electron Beam Melting in the Context of Ni–Al. Metals. 2021; 11(12):2012. https://0-doi-org.brum.beds.ac.uk/10.3390/met11122012
Chicago/Turabian StyleSchaar, Helge, Ingo Steinbach, and Marvin Tegeler. 2021. "Numerical Study of Epitaxial Growth after Partial Remelting during Selective Electron Beam Melting in the Context of Ni–Al" Metals 11, no. 12: 2012. https://0-doi-org.brum.beds.ac.uk/10.3390/met11122012