Gold Bioleaching from Printed Circuit Boards of Mobile Phones by Aspergillus niger in a Culture without Agitation and with Glucose as a Carbon Source
Abstract
:1. Introduction
2. Materials and Methods
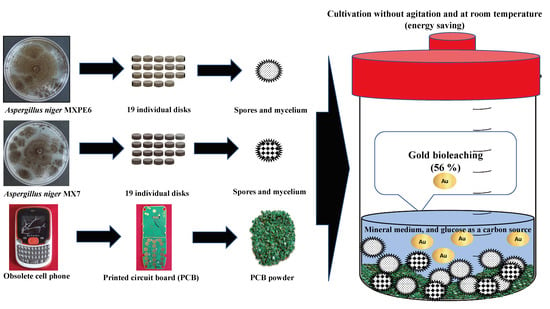
2.1. Fungal Isolates
2.2. Dismantling Cell Phones and Quantification of Au in PCB
2.3. Cultivation Conditions
2.4. Accumulation of Au in Fungal Biomass
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Fungal Growth in Culture Media with or without PCB
3.2. Changes in pH of Culture Media
3.3. Gold Bioleaching by the Aspergillus niger Strains from PCB Residue
3.4. Gold Accumulation in Fungal Biomass
4. Conclusions
5. Recommendations and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Doctori-Blass, V. The economics of cell phone reuse and recycling. Int. J. Adv. Manuf. Technol. 2010, 47, 515–525. [Google Scholar] [CrossRef]
- Ongondo, F.O.; Williams, I.D. Greening academia: Use and disposal of mobile phones among university students. Waste Manag. 2011, 31, 1617–1634. [Google Scholar] [CrossRef] [PubMed]
- Polák, M.; Drápalová, L. Estimation of end of life mobile phones generation: The case study of the Czech Republic. Waste Manag. 2012, 32, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; McPherson, D. Cellular phone E-waste. J. OCEESA 2003, 20, 36–41. [Google Scholar]
- Yamane, L.H.; Tavares de Moraes, V.; Romano-Espinosa, D.C.; Soares-Tenório, J.A. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef]
- Hall, W.J.; Williams, P.T. Separation and recovery of materials from scrap printed circuit boards. Resour. Conserv. Recycl. 2007, 51, 691–709. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Qiu, K. A new technology for recycling materials from waste printed circuit boards. J. Hazard. Mater. 2010, 175, 823–828. [Google Scholar] [CrossRef]
- Park, Y.J.; Fray, D.J. Recovery of high purity precious metals from printed circuit boards. J. Hazard. Mater. 2009, 164, 1152–1158. [Google Scholar] [CrossRef]
- Oguchi, M.; Murakami, S.; Sakanakura, H.; Kida, A.; Kameya, T. A preliminary categorization of end-of-life electrical and electronic equipment as secondary metal resources. Waste Manag. 2011, 31, 2150–2160. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Gramatyka, P.; Nowosielski, R.; Sakiewicz, P. Recycling of waste electrical and electronic equipment. J. Achiev. Mater. Manuf. Eng. 2007, 20, 535–538. [Google Scholar]
- Zhang, L.; Xu, Z. A review of current progress of recycling technologies for metals from waste electrical and electronic equipment. J. Clean. Prod. 2016, 127, 19–36. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, Z. Recycling of non-metallic fractions from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2014, 34, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2013, 33, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, A.; Rhamdhani, M.A.; Brooks, G.; Masood, S. Metal extraction processes for electronic waste and existing industrial routes: A review and Australian perspective. Resources 2014, 3, 152–179. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A. Extraction of metals from electronic waste by bacterial leaching. Environ. Prot. Eng. 2013, 39, 197–208. [Google Scholar]
- Zhu, N.; Xiang, Y.; Zhang, T.; Wu, P.; Dan, Z.; Li, P.; Wu, J. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria. J. Hazard. Mater. 2011, 192, 614–619. [Google Scholar] [CrossRef]
- Gu, W.; Bai, J.; Dai, J.; Zhang, C.; Yuan, W.; Wang, J.; Wang, P.; Zhao, X. Characterization of extreme acidophile bacteria (Acidithiobacillus ferrooxidans) bioleaching copper from flexible PCB by ICP-AES. J. Spectrosc. 2014, 8, 2014269351. [Google Scholar] [CrossRef]
- Olson, G.J.; Brierley, J.A.; Brierley, C.L. Bioleaching review part B: Progress in bioleaching: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Gholami, R.; Borghei, S.; Mousavi, S. Bacterial leaching of a spent Mo-Co-Ni refinery catalyst using Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Hydrometallurgy 2011, 106, 26–31. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Xu, J.; Liang, B. Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J. Hazard. Mater. 2009, 172, 1100–1105. [Google Scholar] [CrossRef]
- Chi, T.D.; Lee, J.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of gold and cooper from waste mobile phone PCB by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 219–222. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.P. Pretreatment of e-waste and mutation of alkali-tolerant cyanogenic bacteria promote gold biorecovery. Bioresour. Technol. 2014, 152, 80–85. [Google Scholar] [CrossRef]
- Tay, S.B.; Natarajan, G.; Rahim, M.N.B.A.; Tan, H.T.; Chung, M.C.M.; Ting, Y.P.; Yew, W.S. Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci. Rep. 2014, 3, 2236. [Google Scholar] [CrossRef]
- Pham, V.A. Gold bioleaching of electronic scrap material by cyanogenic bacteria and its enhancement with biooxidation. Master Thesis, University of Singapore, Singapore, 2009. [Google Scholar]
- Pradhan, J.; Kumar, S. Metals bioleaching from electronic waste by Chromobacterium violaceum and Pseudomonads Sp. Waste Manag. Res. 2012, 30, 1151–1159. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M. Enhancement of simultaneous gold and copper extraction from computer printed circuit boards using Bacillus megaterium. Bioresour. Technol. 2015, 175, 315–324. [Google Scholar] [CrossRef]
- Madrigal-Arias, J.E.; Argumedo-Delira, R.; Alarcón, A.; Mendoza-López, M.R.; García-Barradas, O.; Cruz-Sánchez, J.S.; Ferrera-Cerrato, R.; Jiménez-Fernández, M. Bioleaching of gold, copper and nickel from waste mobile phone PCB and computer goldfinger motherboards by two Aspergillus niger strains. Braz. J. Microbiol. 2015, 46, 707–713. [Google Scholar] [CrossRef]
- Gutiérrez-Rojas, M.; Córdova, J.; Auria, R.; Revah, S.; Favela-Torres, E. Citric acid and polyols production by Aspergillus niger at high glucose concentration in solid state fermentation on inert support. Biotechnol. Lett. 1995, 17, 219–224. [Google Scholar] [CrossRef]
- Papagiannia, M.; Matteya, M.; Kristiansen, B. The influence of glucose concentration on citric acid production and morphology of Aspergillus niger in batch and culture. Enzym. Microb. Technol. 1999, 25, 710–717. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Biochem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Koch, A.L. The kinetics of mycelial growth. J. Gen. Microbiol. 1975, 89, 209–216. [Google Scholar] [CrossRef]
- SAS Institute Inc. The SAS System for Windows, version 9.4; SAS Institute Inc.: Cary, NC, USA, 2017. [Google Scholar]
- Cooke, W.B. Carbon/nitrogen relationships of fungus culture media. Mycopathologia 1968, 34, 305–316. [Google Scholar] [CrossRef]
- Wainwright, M. Metabolic diversity of fungi in relation to growth and mineral cycling in soil—A review. Trans. Br. Mycol. Soc. 1988, 90, 159–170. [Google Scholar] [CrossRef]
- Grewal, H.S.; Kalra, K.L. Fungal production of citric acid. Biotechnol. Adv. 1995, 13, 209–234. [Google Scholar] [CrossRef]
- Legisa, M.; Gradisnik-Grapulin, M. Sudden substrate dilution induces a higher rate of citric acid production by Aspergillus niger. Appl. Environ. Microbiol. 1995, 61, 2732–2737. [Google Scholar]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hidrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Pham, V.A.; Ting, Y.P. Gold bioleaching of electronic waste by cyanogenic bacteria and its enhancement with bio-oxidation. Adv. Mater. Res. 2009, 71–73, 661–664. [Google Scholar] [CrossRef]
- Ren, W.; Li, P.; Geng, Y.; Li, X. Biological leaching of heavy metals from a contaminated soil by Aspergillus niger. J. Hazard. Mater. 2009, 167, 164–169. [Google Scholar] [CrossRef]
- Rezza, I.; Salinas, E.; Elorza, M.; Sanz de Tosetti, M.; Donati, E. Mechanisms involved in bioleaching of an aluminosilicate by heterotrophic microorganisms. Process Biochem. 2001, 36, 495–500. [Google Scholar] [CrossRef]
- Sayer, J.A.; Gadd, G.M. Binding of cobalt and zinc by organic acids and culture filtrates of Aspergillus niger grown in the absence or presence of insoluble cobalt or zinc phosphate. Mycol. Res. 2001, 105, 1261–1267. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Kamali, M.; Gibbs, B.F. Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J. Hazard. Mater. 2004, 110, 77–84. [Google Scholar] [CrossRef]
- Büchs, J. Introduction to advantages and problems of shaken cultures. Biochem. Eng. J. 2001, 7, 91–98. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Brown, R. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Hu, W.; Li, Y. Biodegradation mechanisms and kinetics of azo dye 4BS by a microbial consortium. Chemosphere 2004, 57, 293–301. [Google Scholar] [CrossRef]
- Shih, I.L.; Tsa, K.L.; Hsieh, C. Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem. Eng. J. 2007, 33, 193–201. [Google Scholar] [CrossRef]
- Gomes, N.C.M.; Camargos, E.R.S.; Dias, J.C.T.; Linardi, V.R. Gold and silver accumulation by Aspergillus niger from cyanide-containing solution obtained from the gold mining industry. World J. Microbiol. Biotechnol. 1997, 14, 149. [Google Scholar] [CrossRef]
- Ha, V.H.; Lee, J.C.; Jeong, J.; Hai, H.T.; Jha, M.K. Thiosulfate leaching of gold from waste mobile phones. J. Hazard. Mater. 2010, 178, 1115–1119. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, M.S.; Lee, J.C.; Pandey, B. Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J. Hazard. Mater. 2011, 198, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ficeriová, J.; Baláž, P.; Gock, E. Leaching of gold, silver and accompanying metals from circuit boards (PCBs) waste. Acta Montan. Slovaca 2011, 16, 128–131. [Google Scholar]
- Li, J.Y.; Xu, X.L.; Liu, W.Q. Thiourea leaching gold and silver from the printed circuit boards of waste mobile phones. Waste Manag. 2012, 32, 1209–1212. [Google Scholar]
- Li, J.; Liang, C.; Ma, C. Bioleaching of gold from waste printed circuit boards by Chromobacterium violaceum. J. Mater. Cycles Waste Manag. 2015, 17, 529–539. [Google Scholar] [CrossRef]






| Treatments | YX/S (mg∙g−1) | YCAu/S (mg∙g−1) | k ((mg∙L−1)1/3 d−1) |
|---|---|---|---|
| A. niger MXPE6 | 6.33 | 0.20 | |
| A. niger MXPE6 + PCB | 7.79 | 0.29 | 0.32 |
| A. niger MXPE6+A. niger MX7 | 32.71 | 0.19 | |
| A. niger MXPE6+A. niger MX7 + PCB | 9.59 | 0.11 | 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argumedo-Delira, R.; Gómez-Martínez, M.J.; Soto, B.J. Gold Bioleaching from Printed Circuit Boards of Mobile Phones by Aspergillus niger in a Culture without Agitation and with Glucose as a Carbon Source. Metals 2019, 9, 521. https://0-doi-org.brum.beds.ac.uk/10.3390/met9050521
Argumedo-Delira R, Gómez-Martínez MJ, Soto BJ. Gold Bioleaching from Printed Circuit Boards of Mobile Phones by Aspergillus niger in a Culture without Agitation and with Glucose as a Carbon Source. Metals. 2019; 9(5):521. https://0-doi-org.brum.beds.ac.uk/10.3390/met9050521
Chicago/Turabian StyleArgumedo-Delira, Rosalba, Mario J. Gómez-Martínez, and Brenda Joan Soto. 2019. "Gold Bioleaching from Printed Circuit Boards of Mobile Phones by Aspergillus niger in a Culture without Agitation and with Glucose as a Carbon Source" Metals 9, no. 5: 521. https://0-doi-org.brum.beds.ac.uk/10.3390/met9050521