An Efficient Approach for Nitrogen Diffusion and Surface Nitriding of Boron-Titanium Modified Stainless Steel Alloy for Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
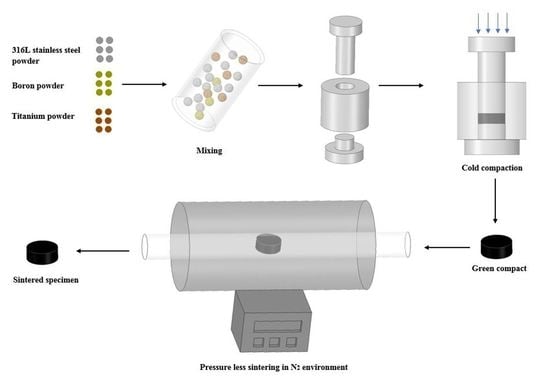
2.1. Specimen Preparation
2.2. Green and Sintered Density Measurement
2.3. Microstructure and Micro Hardness Measurement
2.4. Characterization of Sintered Specimens
2.5. Immersion Testing
2.6. Cytotoxicity Assessment
3. Results
3.1. Green and Sintered Density Measurements
3.2. Microstructure of Sintered Specimens
3.3. Micro Hardness of Specimens
3.4. XRD Analysis of Sintered Specimens
3.5. XPS Analysis of Sintered Specimens
3.6. Immersion Testing in Artificial Saliva Solution
3.7. In Vitro Cytotoxicity Assessment of Sintered Specimens
4. Discussion
5. Conclusions
- 316L stainless steel sintered in nitrogen atmosphere with increased dwell time can help in diffusion of nitrogen into the matrix, thereby forming its respective nitrides as discussed in the XRD and FESEM mapping analysis.
- The sintering parameters helped in formation of a strong nitride layer onto the surface of the samples, as discussed in the XPS analysis. This layer proved to be helpful in the retention of metal ions during weight loss measurements.
- The addition of 2 wt.% titanium addition retained the austenitic structure of the resultant alloy systems, which is important in implant manufacturing. Better results were shown by S5 for nearly all the tests, except for density.
- The corrosion resistance of the alloy systems in artificial saliva solution revealed minimal weight loss with a negligible release of metal ions. This was attributed to the nitrogen which diffused into the matrix and also prepared a strong nitride layer. Both of these results helped in improved corrosion resistance of the alloy systems.
- The cytotoxicity assessment by MTT assay using fibroblast cells indicated that all the alloy systems studied in this research are non-cytotoxic. The SEM images indicate the cell adhesion to the specimen surface, indicating that cells continue their growth. Specimen S5 with 2 wt.% titanium addition exhibited better results than the others, showing more antibacterial properties and indicating the highest cell proliferation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiles, P. The surgery of the osteo-arthritic hip. Br. J. Surg. 1958, 45, 488–497. [Google Scholar] [CrossRef]
- Wiles, P. The classic: The surgery of the osteo-arthritic hip. Clin. Orthop. Relat. Res. 2003, 417, 3–16. [Google Scholar]
- Lo, K.; Shek, C.H.; Lai, J.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.M.A.; Altaf, K.; Baig, Z. Investigation of Boron addition and compaction pressure on the compactibility, densification and microhardness of 316L Stainless Steel. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Disegi, J.; Eschbach, L. Stainless steel in bone surgery. Injury 2000, 31, D2–D6. [Google Scholar] [CrossRef]
- Dewidar, M. Influence of processing parameters and sintering atmosphere on the mechanical properties and microstructure of porous 316L stainless steel for possible hard-tissue applications. Int. J. Mech. Mech. Eng. 2012, 12, 10–24. [Google Scholar]
- Ali, S.; Rani, A.M.A.; Altaf, K.; Hussain, P.; Prakash, C.; Hastuty, S.; Rao, T.V.V.L.N.; Subramaniam, K. Investigation of Alloy Composition and Sintering Parameters on the Corrosion Resistance and Microhardness of 316L Stainless Steel Alloy. In Advances in Manufacturing II; Springer: Berlin/Heidelberg, Germany, 2019; pp. 532–541. [Google Scholar]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Sato, K.; Hyun, S.; Nakajima, H. Fabrication and properties of Lotus-type porous nickel-free stainless steel for biomedical applications. Mater. Sci. Eng. C 2008, 28, 44–50. [Google Scholar] [CrossRef]
- Thomann, U.I.; Uggowitzer, P.J. Wear–corrosion behavior of biocompatible austenitic stainless steels. Wear 2000, 239, 48–58. [Google Scholar] [CrossRef]
- Kuroda, D.; Hiromoto, S.; Hanawa, T.; Katada, Y. Corrosion Behavior of Nickel-Free High Nitrogen Austenitic Stainless Steel in Simulated Biological Environments. Mater. Trans. 2002, 43, 3100–3104. [Google Scholar] [CrossRef]
- Sumita, M.; Hanawa, T.; Teoh, S. Development of nitrogen-containing nickel-free austenitic stainless steels for metallic biomaterials—Review. Mater. Sci. Eng. C 2004, 24, 753–760. [Google Scholar] [CrossRef]
- Hallab, N. Metal Sensitivity in Patients with Orthopedic Implants. JCR: J. Clin. Rheumatol. 2001, 7, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Cenni, E.; Tigani, D.; Trisolino, G.; Baldini, N.; Giunti, A. Sensitivity to implant materials in patients with total knee arthroplasties. Biomaterials 2008, 29, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Berglund, M.; Åkesson, A.; Liden, C. Metals and women’s health. Environ. Res. 2002, 88, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ren, Y. Nickel-free austenitic stainless steels for medical applications. Sci. Technol. Adv. Mater. 2010, 11, 14105. [Google Scholar] [CrossRef] [PubMed]
- Foussereau, J.; Laugier, P. Allergic eczemas from metallic foreign bodies. Trans. St. John’s Hosp. Dermatol. Soc. 1966, 52, 220–225. [Google Scholar]
- Gao, X.; He, R.-X.; Yan, S.-G.; Wu, L.-D. Dermatitis Associated with Chromium Following Total Knee Arthroplasty. J. Arthroplast. 2011, 26, 665.e13–665.e16. [Google Scholar] [CrossRef]
- Beythien, C.; Gutensohn, K.; Fenner, T. Diamond-like carbon coating of coronary stents: Influence on platelet activation, smooth muscle cells and release of metal ions. Eur. Heart J. 1998, 19, 1947. [Google Scholar]
- Menzel, J.; Kirschner, W.; Stein, G. High nitrogen containing Ni-free austenitic steels for medical applications. ISIJ Int. 1996, 36, 893–900. [Google Scholar] [CrossRef]
- Uggowitzer, P.J.; Magdowski, R.; Speidel, M.O. Nickel free high nitrogen austenitic steels. ISIJ Int. 1996, 36, 901–908. [Google Scholar] [CrossRef]
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elements Med. Boil. 2017, 40, 30–36. [Google Scholar] [CrossRef]
- Mjöberg, B.; Hellquist, E.; Mallmin, H.; Lindh, U. Aluminum, Alzheimer’s disease and bone fragility. Acta Orthop. Scand. 1997, 68, 511–514. [Google Scholar] [CrossRef]
- Bayon, R.; Igartua, A.; González, J.; De Gopegui, U.R. Influence of the carbon content on the corrosion and tribocorrosion performance of Ti-DLC coatings for biomedical alloys. Tribol. Int. 2015, 88, 115–125. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Manam, N.; Harun, W.; Shri, D.; Ghani, S.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Sulima, I.; Jaworska, L.; Karwan-Baczewska, J. Effect of Boron Sinter-Aid on the Microstructure and Properties of Austenitic Stainless Steel-TIB2 Composites/Wpływ Dodatku Boru Na Mikrostrukturę IW Łaściwosci Kompozytów Stal Austenityczna-TIB2. Arch. Metall. Mater. 2015, 60, 2619–2624. [Google Scholar] [CrossRef]
- Bayraktaroglu, E.; Gulsoy, H.O.; Gulsoy, N.; Er, O.; Kilic, H. Effect of boron addition on injection molded 316L stainless steel: Mechanical, corrosion properties and in vitro bioactivity. Bio-Med. Mater. Eng. 2012, 22, 333–349. [Google Scholar]
- Szewczyk-Nykiel, A. The effect of the addition of boron on the densification, microstructure and properties of sintered 17-4 PH stainless steel. Czasopismo Techniczne 2014. [Google Scholar] [CrossRef]
- Mishnaevsky, L., Jr.; Levashov, E.; Valiev, R.Z.; Segurado, J.; Sabirov, I.; Enikeev, N.; Prokoshkin, S.; Solov’yov, A.V.; Korotitskiy, A.; Gutmanas, E.; et al. Nanostructured titanium-based materials for medical implants: Modeling and development. Mater. Sci. Eng. R Rep. 2014, 81, 1–19. [Google Scholar] [CrossRef]
- Correa, D.R.N.; Vicente, F.; Donato, T.; Arana-Chavez, V.; Buzalaf, M.; Grandini, C.R. The effect of the solute on the structure, selected mechanical properties, and biocompatibility of Ti–Zr system alloys for dental applications. Mater. Sci. Eng. C 2014, 34, 354–359. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Beline, T.; Ribeiro, A.L.R.; Rangel, E.C.; Da Cruz, N.C.; Landers, R.; Faverani, L.P.; Vaz, L.G.; Fais, L.M.; Vicente, F.B.; et al. Development of binary and ternary titanium alloys for dental implants. Dent. Mater. 2017, 33, 1244–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shemtov-Yona, K.; Rittel, D. On the mechanical integrity of retrieved dental implants. J. Mech. Behav. Biomed. Mater. 2015, 49, 290–299. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Barão, V.A.; Barao, V.A.R. Is there scientific evidence favoring the substitution of commercially pure titanium with titanium alloys for the manufacture of dental implants? Mater. Sci. Eng. C 2017, 71, 1201–1215. [Google Scholar] [CrossRef]
- Balachandran, G. High Nitrogen Steels and Stainless Steels—Manufacturing Properties and Applications; Alpha Science International: Pangbourne, UK, 2004; pp. 40–93. [Google Scholar]
- Tsuchiyama, T.; Uchida, H.; Kataoka, K.; Takaki, S. Fabrication of Fine-grained High Nitrogen Austenitic Steels through Mechanical Alloying Treatment. ISIJ Int. 2002, 42, 1438–1443. [Google Scholar] [CrossRef]
- Kurgan, N. Effects of sintering atmosphere on microstructure and mechanical property of sintered powder metallurgy 316L stainless steel. Mater. Des. 2013, 52, 995–998. [Google Scholar] [CrossRef]
- Saller, G.; Spiradek-Hahn, K.; Scheu, C.; Clemens, H. Microstructural evolution of Cr–Mn–N austenitic steels during cold work hardening. Mater. Sci. Eng. A 2006, 427, 246–254. [Google Scholar] [CrossRef]
- Chao, Z.; Yaomu, X.; Chufeng, L.; Conghua, L. The effect of mucin, fibrinogen and IgG on the corrosion behavior of Ni–Ti alloy and stainless steel. Biometals 2017, 30, 367–377. [Google Scholar] [CrossRef]
- Hussein, M.A.; Yilbas, B.; Kumar, A.M.; Drew, R.; Al-Aqeeli, N. Influence of Laser Nitriding on the Surface and Corrosion Properties of Ti-20Nb-13Zr Alloy in Artificial Saliva for Dental Applications. J. Mater. Eng. Perform. 2018, 27, 4655–4664. [Google Scholar] [CrossRef]
- Das, K.K.; Das, S.N.; Dhundasi, S.A. Nickel, its adverse health effects & oxidative stress. Indian J. Med. Res. 2008, 128, 412. [Google Scholar]










| Element | C | Si | O | Mn | Ni | Cr | Mo | Fe |
|---|---|---|---|---|---|---|---|---|
| wt.% | 0.028 | 0.9 | 0.068 | 1.5 | 12.01 | 17.04 | 2.4 | Balance |
| S.No | Alloy | Composition |
|---|---|---|
| 1 | S1 | Pure 316L SS |
| 2 | S2 | 316L SS + 0.25 B + 0.5 Ti |
| 3 | S3 | 316L SS + 0.25 B + 1 Ti |
| 4 | S4 | 316L SS + 0.25 B + 1.5 Ti |
| 5 | S5 | 316L SS + 0.25 B + 2 Ti |
| S.No | Alloy | Green Density | Sintered Density | Densification |
|---|---|---|---|---|
| 1 | S1 | 6.500 g/cm3 | 7.575 g/cm3 | 95.88% |
| 2 | S2 | 6.385 g/cm3 | 7.387 g/cm3 | 93.50% |
| 3 | S3 | 6.212 g/cm3 | 7.139 g/cm3 | 90.36% |
| 4 | S4 | 6.116 g/cm3 | 7.008 g/cm3 | 88.70% |
| 5 | S5 | 6.002 g/cm3 | 6.899 g/cm3 | 87.32% |
| S.No | Alloy | Weight before Immersion | Weight after Immersion | Δm (g) |
|---|---|---|---|---|
| 1 | S1 | 17.310 g | 17.306 g | 0.004 g |
| 2 | S2 | 18.220 g | 18.217 g | 0.003 g |
| 3 | S3 | 18.250 g | 18.248 g | 0.002 g |
| 4 | S4 | 17.240 g | 18.237 g | 0.003 g |
| 5 | S5 | 18.300 g | 18.299 g | 0.001 g |
| S.No | Alloy | Elements Concentration (ppm) | ||
|---|---|---|---|---|
| Fe | Cr | Ni | ||
| 1 | S1 | 0.001 | 0.000 | 0.050 |
| 2 | S2 | 0.003 | 0.001 | 0.090 |
| 3 | S3 | 0.009 | 0.001 | 0.080 |
| 4 | S4 | 0.010 | 0.003 | 0.040 |
| 5 | S5 | 0.000 | 0.000 | 0.050 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Abdul Rani, A.M.; Mufti, R.A.; Hastuty, S.; Hussain, M.; Shehzad, N.; Baig, Z.; Abdu Aliyu, A.A. An Efficient Approach for Nitrogen Diffusion and Surface Nitriding of Boron-Titanium Modified Stainless Steel Alloy for Biomedical Applications. Metals 2019, 9, 755. https://0-doi-org.brum.beds.ac.uk/10.3390/met9070755
Ali S, Abdul Rani AM, Mufti RA, Hastuty S, Hussain M, Shehzad N, Baig Z, Abdu Aliyu AA. An Efficient Approach for Nitrogen Diffusion and Surface Nitriding of Boron-Titanium Modified Stainless Steel Alloy for Biomedical Applications. Metals. 2019; 9(7):755. https://0-doi-org.brum.beds.ac.uk/10.3390/met9070755
Chicago/Turabian StyleAli, Sadaqat, Ahmad Majdi Abdul Rani, Riaz Ahmad Mufti, Sri Hastuty, Murid Hussain, Nasir Shehzad, Zeeshan Baig, and Abdul Azeez Abdu Aliyu. 2019. "An Efficient Approach for Nitrogen Diffusion and Surface Nitriding of Boron-Titanium Modified Stainless Steel Alloy for Biomedical Applications" Metals 9, no. 7: 755. https://0-doi-org.brum.beds.ac.uk/10.3390/met9070755