Directed Irradiation Synthesis as an Advanced Plasma Technology for Surface Modification to Activate Porous and “as-received” Titanium Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacturing of Porous and Non-Porous c.p. Ti Samples
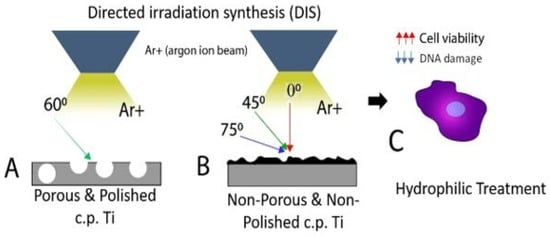
2.2. Directed Irradiation Synthesis (DIS) of Porous and Non-Porous c.p. Ti Samples
2.3. Structural and Surface Free Energy Characterization of c.p. Ti Samples Modified by DIS
2.4. Biological Evaluation of c.p. Ti Samples Modified by DIS
2.5. Statistical Analyses
3. Results
3.1. Structural Topography Characterization of As-Received (AR) and DIS Treated c.p. Ti Samples
3.2. Surface Free Energy Evaluation of As-Received (AR) and Porous DIS Treated c.p. Ti Samples
3.3. Biological Assessment of c.p. Ti Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nazarian, A.; von Stechow, D.; Zurakowski, D.; Müller, R.; Snyder, B.D. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif. Tissue Int. 2008, 83, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, 96–101. [Google Scholar]
- Busscher, H.J.; Van Der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; Van Den Dungen, J.J.; Zaat, S.A.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, P.G.; Jimbo, R.; Bonfante, E.A. Experimental and clinical knowledge of nanometer scale designing on endosteal implants. In Implant Surfaces and Their Biological and Clinical Impact; Springer: Berlin/Heidelberg, Germany, 2015; pp. 29–43. [Google Scholar]
- Koldsland, O.C.; Scheie, A.A.; Aass, A.M. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J. Periodontol. 2010, 81, 231–238. [Google Scholar] [CrossRef]
- Materials Surface Effects on Biological Interactions|SpringerLink. Available online: https://0-link-springer-com.brum.beds.ac.uk/chapter/10.1007/978-90-481-8790-4_12 (accessed on 29 October 2018).
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Oppenheimer, S.M.; Dunand, D.C. Porous NiTi by creep expansion of argon-filled pores. Mater. Sci. Eng. A 2009, 523, 70–76. [Google Scholar] [CrossRef]
- Chino, Y.; Dunand, D.C. Directionally freeze-cast titanium foam with aligned, elongated pores. Acta Mater. 2008, 56, 105–113. [Google Scholar] [CrossRef]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low stiffness porous Ti structures for load-bearing implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef]
- An, Y.B.; Oh, N.H.; Chun, Y.W.; Kim, Y.H.; Kim, D.K.; Park, J.S.; Kwon, J.J.; Choi, K.O.; Eom, T.G.; Byun, T.H. Mechanical properties of environmental-electro-discharge-sintered porous Ti implants. Mater. Lett. 2005, 59, 2178–2182. [Google Scholar] [CrossRef]
- Orru, R.; Licheri, R.; Locci, A.M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mater. Sci. Eng. R Rep. 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Torres, Y.; Pavón, J.J.; Nieto, I.; Rodríguez, J.A. Conventional powder metallurgy process and characterization of porous titanium for biomedical applications. Metall. Mater. Trans. B 2011, 42, 891–900. [Google Scholar] [CrossRef]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.; Rodriguez, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C 2014, 37, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.E.; Mabuchi, M.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T. Processing of biocompatible porous Ti and Mg. Scr. Mater. 2001, 45, 1147–1153. [Google Scholar] [CrossRef]
- Oh, I.-H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Boyan, B.D. Modulation of osteogenesis via implant surface design. In Bone Engineering; EM Squared Incorporated: Toronto, ON, Canada, 2000; pp. 232–239. [Google Scholar]
- Boyan, B.D.; Cheng, A.; Olivares-Navarrete, R.; Schwartz, Z. Implant surface design regulates mesenchymal stem cell differentiation and maturation. Adv. Dent. Res. 2016, 28, 10–17. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef]
- Dalby, M.J. Cellular response to low adhesion nanotopographies. Int. J. Nanomed. 2007, 2, 373–381. [Google Scholar]
- Biggs, M.J.P.; Richards, R.G.; Dalby, M.J. Nanotopographical modification: A regulator of cellular function through focal adhesions. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.; Lauer, J.; Selig, M.; Hanak, M.; Walters, B.; Rolauffs, B. Shaping the Cell and the Future: Recent Advancements in Biophysical Aspects Relevant to Regenerative Medicine. J. Funct. Morphol. Kinesiol. 2018, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Abagnale, G.; Steger, M.; Nguyen, V.H.; Hersch, N.; Sechi, A.; Joussen, S.; Denecke, B.; Merkel, R.; Hoffmann, B.; Dreser, A.; et al. Surface topography enhances differentiation of mesenchymal stem cells towards osteogenic and adipogenic lineages. Biomaterials 2015, 61, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, T.; Dalby, M.J.; Hart, A.; Tare, R.; Oreffo, R.O.; Su, B. Fabrication of pillar-like titania nanostructures on titanium and their interactions with human skeletal stem cells. Acta Biomater. 2009, 5, 1433–1441. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Allain, J.P.; Nieto, M.; Hendricks, M.R.; Plotkin, P.; Harilal, S.S.; Hassanein, A. IMPACT: A facility to study the interaction of low-energy intense particle beams with dynamic heterogeneous surfaces. Rev. Sci. Instrum. 2007, 78, 113105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schernthaner, M.; Reisinger, B.; Wolinski, H.; Kohlwein, S.D.; Trantina-Yates, A.; Fahrner, M.; Romanin, C.; Itani, H.; Stifter, D.; Leitinger, G. Nanopatterned polymer substrates promote endothelial proliferation by initiation of β-catenin transcriptional signaling. Acta Biomater. 2012, 8, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Tserepi, A.; Gogolides, E.; Bourkoula, A.; Kanioura, A.; Kokkoris, G.; Petrou, P.S.; Kakabakos, S.E. Plasma nanotextured polymeric surfaces for controlling cell attachment and proliferation: A short review. Plasma Chem. Plasma Process. 2016, 36, 107–120. [Google Scholar] [CrossRef]
- Sjöström, T.; McNamara, L.E.; Meek, R.D.; Dalby, M.J.; Su, B. 2D and 3D nanopatterning of titanium for enhancing osteoinduction of stem cells at implant surfaces. Adv. Healthc. Mater. 2013, 2, 1285–1293. [Google Scholar] [CrossRef]
- Lu, J.; Rao, M.P.; MacDonald, N.C.; Khang, D.; Webster, T.J. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008, 4, 192–201. [Google Scholar] [CrossRef]
- ASTM F67-00 (2002). Standard Specification for Unalloyed Titanium for Surgical Implant Applications; American Society for Testing and Materials: Philadephia, PA, USA, 2002. [Google Scholar]
- Civantos, A.; Barnwell, A.; Shetty, A.R.; Pavón, J.J.; El-Atwani, O.; Arias, S.L.; Lang, E.; Reece, L.M.; Chen, M.; Allain, J.P. Designing Nanostructured Ti6Al4V Bioactive Interfaces with Directed Irradiation Synthesis toward Cell Stimulation to Promote Host—Tissue-Implant Integration. ACS Biomater. Sci. Eng. 2019, 5, 3325–3339. [Google Scholar] [CrossRef]
- Allain, J.P.; Shetty, A. Unraveling atomic-level self-organization at the plasma-material interface. J. Phys. D Appl. Phys. 2017, 50, 283002. [Google Scholar] [CrossRef]
- Chan, W.L.; Pavenayotin, N.; Chason, E. Kinetics of ion-induced ripple formation on Cu(001) surfaces. Phys. Rev. B 2004, 69. [Google Scholar] [CrossRef]
- Qian, H.X.; Zhou, W. Self-organization of ripples on Ti irradiated with focused ion beam. Appl. Surf. Sci. 2012, 258, 1924–1928. [Google Scholar] [CrossRef]
- Busse, C.; Engin, C.; Hansen, H.; Linke, U.; Michely, T.; Urbassek, H.M. Adatom formation and atomic layer growth on Al (1 1 1) by ion bombardment: Experiments and molecular dynamics simulations. Surf. Sci. 2001, 488, 346–366. [Google Scholar] [CrossRef]
- Naumann, J.; Osing, J.; Quinn, A.J.; Shvets, I.V. Morphology of sputtering damage on Cu(111) studied by scanning tunneling microscopy. Surf. Sci. 1997, 388, 212–219. [Google Scholar] [CrossRef]
- Costantini, G.; Rusponi, S.; de Mongeot, F.B.; Boragno, C.; Valbusa, U. Periodic structures induced by normal-incidence sputtering on Ag(110) and Ag(001): Flux and temperature dependence. J. Phys. Condens. Matter 2001, 13, 5875. [Google Scholar] [CrossRef]
- Malis, O.; Brock, J.D.; Headrick, R.L.; Yi, M.-S.; Pomeroy, J.M. Ion-induced pattern formation on Co surfaces: An x-ray scattering and kinetic Monte Carlo study. Phys. Rev. B 2002, 66. [Google Scholar] [CrossRef] [Green Version]
- Murty, M.R.; Curcic, T.; Judy, A.; Cooper, B.H.; Woll, A.R.; Brock, J.D.; Kycia, S.; Headrick, R.L. X-ray scattering study of the surface morphology of Au(111) during Ar+ ion irradiation. Phys. Rev. Lett. 1998, 80. [Google Scholar] [CrossRef]
- Kalff, M.; Comsa, G.; Michely, T. Temperature dependent morphological evolution of Pt (1 1 1) by ion erosion: Destabilization, phase coexistence and coarsening. Surf. Sci. 2001, 486, 103–135. [Google Scholar] [CrossRef]
- Karmakar, P.; Ghose, D. Ion beam sputtering induced ripple formation in thin metal films. Surf. Sci. 2004, 554, 101–106. [Google Scholar] [CrossRef] [Green Version]
- van Dijken, S.; de Bruin, D.; Poelsema, B. Kinetic physical etching for versatile novel design of well ordered self-affine nanogrooves. Phys. Rev. Lett. 2001, 86, 4608–4611. [Google Scholar] [CrossRef]
- Luttrell, T.; Batzill, M. Nanoripple formation on TiO2(110) by low-energy grazing incidence ion sputtering. Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef]
- Hino, T.; Nakai, T.; Nishikawa, M.; Hirohata, Y.; Yamauchi, Y. Smoothing of polycrystalline copper with rough surface by oblique argon-ion irradiation. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2006, 24, 1918–1921. [Google Scholar] [CrossRef] [Green Version]
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- Sá, J.C.; de Brito, R.A.; Moura, C.E.; Silva, N.B.; Alves, M.; Júnior, C.A. Influence of argon-ion bombardment of titanium surfaces on the cell behavior. Surf. Coat. Technol. 2009, 203, 1765–1770. [Google Scholar] [CrossRef]
- Ryzhkin, I.A.; Petrenko, V.F. Physical mechanisms responsible for ice adhesion. J. Phys. Chem. B 1997, 101, 6267–6270. [Google Scholar] [CrossRef]
- Riedel, N.A.; Williams, J.D.; Popat, K.C. Ion beam etching titanium for enhanced osteoblast response. J. Mater. Sci. 2011, 46, 6087–6095. [Google Scholar] [CrossRef]
- Sigmund, P. Theory of sputtering. I. Sputtering yield of amorphous and polycrystalline targets. Phys. Rev. 1969, 184. [Google Scholar] [CrossRef]
- Bradley, R.M.; Harper, J.M. Theory of ripple topography induced by ion bombardment. J. Vac. Sci. Technol. A Vac. Surf. Film. 1988, 6, 2390–2395. [Google Scholar] [CrossRef]
- Vajo, J.J.; Doty, R.E.; Cirlin, E.-H. Influence of O+2 energy, flux, and fluence on the formation and growth of sputtering-induced ripple topography on silicon. J. Vac. Sci. Technol. A Vac. Surf. Film. 1996, 14, 2709–2720. [Google Scholar] [CrossRef]
- Li, J.; Stein, D.; McMullan, C.; Branton, D.; Aziz, M.J.; Golovchenko, J.A. Ion-beam sculpting at nanometre length scales. Nature 2001, 412, 166–169. [Google Scholar] [CrossRef] [Green Version]
- Dotan, A.; Dodiuk, H.; Laforte, C.; Kenig, S. The relationship between water wetting and ice adhesion. J. Adhes. Sci. Technol. 2009, 23, 1907–1915. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]








| Samples | Energy (KeV) | Gas Species (ions) | Fluence (×1017, cgs) | Incidence Angle (°) |
|---|---|---|---|---|
| PPS1 | 1 | Argon | 1.0 | 60 |
| PPS2 | 1 | Argon | 2.5 | 60 |
| NPS1 | 0.5 | Argon | 5.0 | 0 |
| NPS2 | 0.5 | Argon | 5.0 | 45 |
| NPS3 | 0.5 | Argon | 3.5 | 75 |
| Samples | DIS-Incidence Angle (°) | Roughness RMS (nm) | Average Height (nm) | Contact Angle (°) |
|---|---|---|---|---|
| c.p. Ti | -- | 0.93 | 1.7 | 53.0 ± 0.6 |
| PPS1# | -- | 0.32 | 2.6 | 51.1 ± 2.7 |
| PPS2# | -- | 0.08 | 0.5 | 55.5 ± 3.1 |
| NPS | -- | * | * | 65.9 ± 4.3 |
| PPS1 | 60 | 0.57 | 6.7 | 13.4 ± 4.5 |
| PPS2 | 60 | 3.49 | 18.1 | 28.1 ± 5.2 |
| NPS1 | 0 | 87.0 | 172.0 | 33.3 ± 3.5 |
| NPS2 | 45 | 73.35 | 185.0 | 32.1 ± 4.2 |
| NPS3 | 75 | 22.07 | 52.4 | 25.6 ± 3.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civantos, A.; Allain, J.P.; Pavón, J.J.; Shetty, A.; El-Atwani, O.; Walker, E.; Arias, S.L.; Gordon, E.; Rodríguez-Ortiz, J.A.; Chen, M.; et al. Directed Irradiation Synthesis as an Advanced Plasma Technology for Surface Modification to Activate Porous and “as-received” Titanium Surfaces. Metals 2019, 9, 1349. https://0-doi-org.brum.beds.ac.uk/10.3390/met9121349
Civantos A, Allain JP, Pavón JJ, Shetty A, El-Atwani O, Walker E, Arias SL, Gordon E, Rodríguez-Ortiz JA, Chen M, et al. Directed Irradiation Synthesis as an Advanced Plasma Technology for Surface Modification to Activate Porous and “as-received” Titanium Surfaces. Metals. 2019; 9(12):1349. https://0-doi-org.brum.beds.ac.uk/10.3390/met9121349
Chicago/Turabian StyleCivantos, Ana, Jean Paul Allain, Juan Jose Pavón, Akshath Shetty, Osman El-Atwani, Emily Walker, Sandra L. Arias, Emily Gordon, José A. Rodríguez-Ortiz, Mike Chen, and et al. 2019. "Directed Irradiation Synthesis as an Advanced Plasma Technology for Surface Modification to Activate Porous and “as-received” Titanium Surfaces" Metals 9, no. 12: 1349. https://0-doi-org.brum.beds.ac.uk/10.3390/met9121349