The Diabetic Lung: Insights into Pulmonary Changes in Children and Adolescents with Type 1 Diabetes
Abstract
:1. Introduction
2. Methods
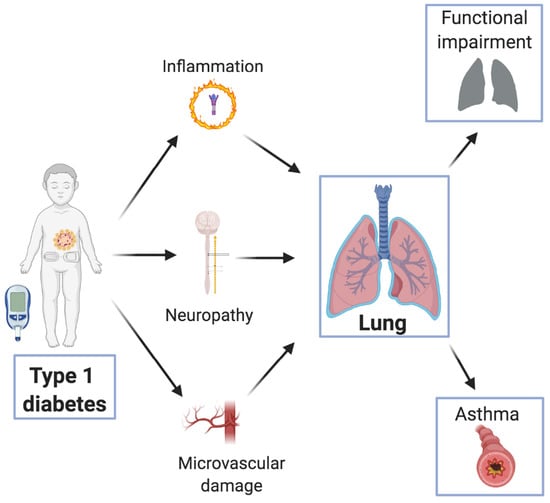
3. Pathogenesis of Diabetes-Induced Lung Damage
4. Pulmonary Function in Children and Adolescents with T1D
4.1. Pulmonary Diffusing Capacity
5. T1D and Asthma
6. Controversial Aspects and Future Directions
6.1. Research Methodology
- The available literature was scarce compared to publications on other diabetes-related complications or subclinical damage in pediatric age.
- The applied research design was not always appropriate. Even though most studies had a control group, 4 were designed without including healthy children and adolescents as controls. Failure to use a control group makes it difficult to draw meaningful conclusions.
- Characteristics of the study population. The age range of the T1D population recruited varied widely between the studies published so far. In particular, the upper age range varied the most, with young adults (up to 22 years of age) also included in pediatric studies. Pubertal stage was not taken into consideration by the majority of the authors. Only 2 research groups adjusted their results according to the pubertal stage.
- Studies at disease onset.
- Currently, only one study explored the pulmonary function at T1D onset. The majority of authors recruited patients some years after disease onset. We believe that including patients at the very beginning of diabetes onset would enable a better understanding of the progression of lung damage during the pediatric age. Prospective studies would surely add new insights into the subclinical progression of lung involvement.
6.2. Disease-Related Variables and Therapy
6.2.1. Indicators of Glycemic Control
6.2.2. Therapy
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.Y. Age of onset of diabetes and all-cause mortality. World J. Diabetes 2020, 11, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef] [Green Version]
- Patterson, C.C.; Dahlquist, G.; Gyürüs, E.; Green, A.; Soltesz, G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–2020: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, C.; Wang, C.; Li, P.; Wang, W.; Ye, J.; Gu, X.; Wang, X.; Shen, S.; Zhi, D.; et al. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: Epidemiology in Shanghai during 1997–2011. Acta Diabetol. 2014, 51, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Imkampe, A.K.; Gulliford, M.C. Trends in Type1 diabetes incidence in the UK in 0- to 14-year-olds and in 15- to 34-year-olds, 1991–2008. Diabet. Med. 2011, 28, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Chobot, P.; Polańska, J.; Szadkowska, A.; Krętowski, A.; Bandurska-Stankiewicz, E.; Ciechanowska, M.; Deja, G.; Myśliwiec, M.; Peczynska, J.; Rutkowska, J.; et al. Rapid increase in the incidence of type 1 diabetes in Polish children from 1989 to 2004, and predictions for 2010 to 2025. Diabetologia 2011, 54, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Skordis, N.; Efstathiou, E.; Kyriakides, T.C.; Savvidou, A.; Savva, S.C.; Phylactou, L.A.; Shammas, C.; Neocleous, V. Epidemiology of type 1 diabetes mellitus in Cyprus: Rising incidence at the dawn of the 21st century. Hormones 2012, 11, 86–93. [Google Scholar] [CrossRef]
- Dahlquist, G.; Mustonen, L. Analysis of 20 years of prospective registration of childhood onset diabetes Time trends and birth cohort effects. Acta Paediatr. Int. J. Paediatr. 2000, 89, 1231–1237. [Google Scholar] [CrossRef]
- Harjutsalo, V.; Sjöberg, L.; Tuomilehto, J. Time trends in the incidence of type 1 diabetes in Finnish children: A cohort study. Lancet 2008, 371, 1777–1782. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Mayer-Davis, E.J. What do we know about the trends in incidence of childhood-onset type 1 diabetes? Diabetologia 2019, 62, 370–372. [Google Scholar] [CrossRef] [Green Version]
- Divers, J.; Mayer-Davis, E.J.; Lawrence, J.M.; Isom, S.; Dabelea, D.; Dolan, L.; Imperatore, G.; Marcovina, S.; Pettitt, D.J.; Pihoker, C.; et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths Selected Counties and Indian Reservations, United States, 2002–2015. Mmwr. Morb. Mortal. Wkly. Rep. 2020, 69, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Mazzantini, S.; Ben Nasr, M.; Fiorina, P.; Scaramuzza, A.E.; Zuccotti, G.V. Explaining the increased mortality in type 1 diabetes. World J. Diabetes 2015, 6, 889. [Google Scholar] [CrossRef] [PubMed]
- Downie, E.; Craig, M.E.; Hing, S.; Cusumano, J.; Chan, A.K.; Donaghue, K.C. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: Role of insulin therapy and glycemic control. Diabetes Care 2011, 34, 2368–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.-M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [Green Version]
- Schuyler, M.R.; Niewoehner, D.E.; Inkley, S.R.; Kohn, R. Abnormal lung elasticity in juvenile diabetes mellitus. Am. Rev. Respir. Dis. 1976, 113, 37–41. [Google Scholar] [CrossRef]
- Davis, T.M.E.; Knuiman, M.; Kendall, P.; Vu, H.; Davis, W.A. Reduced pulmonary function and its associations in type 2 diabetes: The Fremantle Diabetes Study. Diabetes Res. Clin. Pract. 2000, 50, 153–159. [Google Scholar] [CrossRef]
- Pogson, Z.E.K.; McKeever, T.M.; Fogarty, A. The association between serum osmolality and lung function among adults. Eur. Respir. J. 2008, 32, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Scaramuzza, A.E.; Morelli, M.; Rizzi, M.; Borgonovo, S.; De Palma, A.; Mameli, C.; Giani, E.; Beretta, S.; Zuccotti, G.V. Impaired diffusing capacity for carbon monoxide in children with type 1 diabetes: Is this the first sign of long-term complications? Acta Diabetol. 2012, 49, 159–164. [Google Scholar] [CrossRef]
- Martín-Frías, M.; Lamas, A.; Lara, E.; Alonso, M.; Ros, P.; Barrio, R. Pulmonary function in children with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2015, 28, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Cattaneo, C.; Lonoce, L.; Bedogni, G.; Redaelli, F.C.; MacEdoni, M.; Zuccotti, G.V.; Pagliarini, E. Associations among taste perception, food neophobia and preferences in type 1 diabetes children and adolescents: A cross-sectional study. Nutrients 2019, 11, 3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Wu, J.; Jin, Z.; Yan, L.-J. Potential biochemical mechanisms of lung injury in diabetes. Aging Dis. 2017, 8, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsubara, T.; Hara, F. The pulmonary function and histopathological studies of the lung in diabetes mellitus. Nippon Ika Daigaku Zasshi 1991, 58, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Sandler, M. Is the lung a ‘target organ’ in diabetes mellitus? Arch. Intern. Med. 1990, 150, 1385–1388. [Google Scholar] [CrossRef]
- Fuso, L.; Cotroneo, P.; Basso, S.; De Rosa, M.; Manto, A.; Ghirlanda, G.; Pistelli, R. Postural variations of pulmonary diffusing capacity in insulin-dependent diabetes mellitus. Chest 1996, 110, 1009–1013. [Google Scholar] [CrossRef]
- Weynand, B.; Jonckheere, A.; Frans, A.; Rahier, J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration 1999, 66, 14–19. [Google Scholar] [CrossRef]
- Vracko, R.; Thorning, D.; Huang, T.W. Basal lamina of alveolar epithelium and capillaries: Quantitative changes with aging and in diabetes mellitus. Am. Rev. Respir. Dis. 1979, 120, 973–983. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, Z.; Guo, Z.; Zhao, F.; Wang, Y.; Cai, L.; Yang, J. Type 1 diabetes mellitus is an independent risk factor for pulmonary fibrosis. Cell Biochem. Biophys. 2014, 70, 1385–1391. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Naso, F.C.; De Mello, R.N.; Bona, S.; Dias, A.S.; Porawski, M.; Ferraz, A.D.B.F.; Richter, M.F.; Marroni, N.P. Effect of Agaricus blazei Murill on the pulmonary tissue of animals with streptozotocin-induced diabetes. Exp. Diabetes Res. 2010, 2010, 543926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Tan, Y.; Zhao, F.; Ma, Z.; Wang, Y.; Zheng, S.; Epstein, P.N.; Yu, J.; Yin, X.; Zheng, Y.; et al. Angiotensin ii plays a critical role in diabetic pulmonary fibrosis most likely via activation of nadph oxidase-mediated nitrosative damage. Am. J. Physiol. Endocrinol. Metab. 2011, 301, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, Z.; Yan, L.-J. Redox imbalance and mitochondrial abnormalities in the diabetic lung. Redox Biol. 2016, 11, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Durdík, P.; Vojtková, J.; Michnová, Z.; Turčan, T.; Šujanská, A.; Kuchta, M.; Čiljaková, M. Pulmonary function tests in type 1 diabetes adolescents with diabetic cardiovascular autonomic neuropathy. J. Diabetes Complicat. 2016, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Borst, B.V.D.; Gosker, H.R.; Zeegers, M.P.; Schols, A.M.W.J. Pulmonary function in diabetes a metaanalysis. Chest 2010, 138, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, B.; Perejda, A.J.; Sandborg, C.; Kershnar, A.K.; Uitto, J. Skin, Joint, and Pulmonary Changes in Type I Diabetes Mellitus. Am. J. Dis. Child. 1986, 140, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Primhak, R.A.; Whincup, G.; Tsanakas, J.N.; Milner, R.D.G. Reduced vital capacity in insulin-dependent diabetes. Diabetes 1987, 36, 24–326. [Google Scholar] [CrossRef]
- Verrotti, A.; Verini, M.; Chiarelli, F.; Verdesca, V.; Misticoni, G.; Morgese, G. Pulmonary function in diabetic children with and without persistent microalbuminuria. Diabetes Res. Clin. Pract. 1993, 21, 171–176. [Google Scholar] [CrossRef]
- Van Gent, R.; Brackel, H.; De Vroede, M.; Van Der Ent, C. Lung function abnormalities in children with type I diabetes. Respir. Med. 2002, 96, 976–978. [Google Scholar] [CrossRef] [Green Version]
- Cazzato, S.; Bernardi, F.; Salardi, S.; Tassinari, D.; Corsini, I.; Ragni, L.; Cicognani, A.; Cacciari, E. Lung Function in Children with Diabetes Mellitus. Pediatr. Pulmonol. 2004, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Montesano, M.; Barreto, M.; Pagani, J.; Stegagno, M.; Multari, G.; Ronchetti, R. Diffusing capacity for carbon monoxide in children with type 1 diabetes. Diabetologia 2004, 47, 1931–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, I.L.; Saad, K.; Abdel-Azeem, A.; Mohamed, S.; Othman, H.A.; Baseer, K.A.A.; Thabet, A.F.; El-Houfey, A.A. Evaluation of pulmonary function changes in children with type 1 diabetes mellitus in Upper Egypt. Ther. Adv. Endocrinol. Metab. 2015, 6, 87–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mendalawi, M.D.; Al-Saadi, M.M. Lung functions in poorly controlled type 1 Saudi diabetic children and adolescents. Saudi Med. J. 2012, 33, 99–100. [Google Scholar] [PubMed]
- Pieniawska, A.; Horodnicka-Józwa, A.; Petriczko, E.; Walczak, M. Evaluation of respiratory function tests in children and adolescents with type 1 diabetes. Pediatr. Endocrinol. Diabetes. Metab. 2012, 18, 15–20. [Google Scholar]
- Anık, A.; Anık, A.; Uysal, P. Assessment of pulmonary function by impulse oscillometry and spirometry in children with type 1 diabetes mellitus. Pediatr. Pulmonol. 2020. [Google Scholar] [CrossRef]
- Villa, M.P.; Cacciari, E.; Bernardi, F.; Cicognani, A.; Salardi, S.; Zapulla, F. Bronchial Reactivity in Diabetic Patients: Relationship to Duration of Diabetes and Degree of Glycemic Control. Am. J. Dis. Child. 1988, 142, 726–729. [Google Scholar] [CrossRef]
- Vogt, B.W.; Schleicher, E.D.; Wieland, O.H. ε-Amino-Lysine-Bound Glucose in Human Tissues Obtained at Autopsy: Increase in Diabetes Mellitus. Diabetes 1982, 31, 1123–1127. [Google Scholar] [CrossRef] [Green Version]
- Suresh, V.; Reddy, A.; Mohan, A.; Rajgopal, G.; Satish, P.; Harinarayan, C.; Rekha, P.; Sachan, A. High prevalence of spirometric abnormalities in patients with type 1 diabetes mellitus. Pediatr. Endocrinol. Diabetes. Metab. 2011, 17, 71–75. [Google Scholar]
- Ljubić, S.; Metelko, Ž.; Car, N.; Roglić, G.; Dražić, Z. Reduction of diffusion capacity for carbon monoxide in diabetic patients. Chest 1998, 114, 1033–1035. [Google Scholar] [CrossRef]
- Wheatley, C.M.; Baldi, J.C.; Cassuto, N.A.; Foxx-Lupo, W.T.; Snyder, E.M. Glycemic control influences lung membrane diffusion and oxygen saturation in exercise-trained subjects with type 1 diabetes: Alveolar-capillary membrane conductance in type 1 diabetes. Eur. J. Appl. Physiol. 2011, 111, 567–578. [Google Scholar] [CrossRef]
- Atkins, R.C.; Zimmet, P. Diabetic kidney disease: Act now or pay later. Diabetes Res. Clin. Pract. 2010, 90, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Coast, J.R.; Hempleman, S.C.; Baldi, J.C. Type 1 Diabetes Duration Decreases Pulmonary Diffusing Capacity during Exercise. Respiration 2017, 91, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsblom, C.; Sund, R.; Knip, M.; Groop, P.-H. Incidence of type 1 diabetes in Finland. JAMA 2013, 310, 427–428. [Google Scholar] [CrossRef] [Green Version]
- Pearce, N.; Aït-Khaled, N.; Beasley, R.; Mallol, J.; Keil, U.; Mitchell, E.; Robertson, C. Worldwide trends in the prevalence of asthma symptoms: Phase III of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2007, 62, 758–766. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Current Asthma Prevalence Percent Asthma Surveillance Data. National Health Interview Survey (NHIS). 2012. Available online: https://www.cdc.gov/asthma/nhis/2012/data.htm (accessed on 26 January 2021).
- Romagnani, S. Th1 and Th2 in human diseases. Clin. Immunol. Immunopathol. 1996, 80, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.M. Type 1 Diabetes-Associated Autoimmunity: Natural History, Genetic Associations, and Screening. J. Clin. Endocrinol. Metab. 2006, 91, 1210–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.K.; Mehrotra, S.; Agarwal, S.S. The paradigm of Th1 and Th2 cytokines. Immunol. Res. 1999, 20, 147–161. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Hanifin, J.M.; Beck, L.A.; Lemanske, R.F.; Sampson, H.A.; Weiss, S.T.; Leung, D.Y.M. Atopic dermatitis and asthma: Parallels in the evolution of treatment. Pediatrics 2003, 111, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosca, M.A.; Villa, E.; Silvestri, M.; D’Annunzio, G.; Pistorio, A.; Aicardi, M.; Minicucci, L.; Lorini, R.; Rossi, G.A. Discrepancy between sensitization to inhaled allergens and respiratory symptoms in pediatric patients with type 1 diabetes mellitus. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2009, 20, 385–391. [Google Scholar] [CrossRef]
- Jasser-Nitsche, H.; Varga, E.-M.; Borkenstein, H.M.; Höntzsch, J.; Suppan, E.; Weinhandl, G.; Pieringer, L.; Avian, A.; Fröhlich-Reiterer, E. Type 1 diabetes in children and adolescents is not associated with a reduced prevalence of atopy and allergic diseases. Pediatr. Diabetes 2017, 18, 890–894. [Google Scholar] [CrossRef]
- Seiskari, T.; Viskari, H.; Kondrashova, A.; Haapala, A.-M.; Ilonen, J.; Knip, M.; Hyöty, H. Co-occurrence of allergic sensitization and type 1 diabetes. Ann. Med. 2010, 42, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Black, M.H.; Anderson, A.; Bell, R.A.; Dabelea, D.; Pihoker, C.; Saydah, S.; Seid, M.; Standiford, D.A.; Waitzfelder, B.; Marcovina, S.M.; et al. Prevalence of asthma and its association with glycemic control among youth with diabetes. Pediatrics 2011, 128, e839–e847. [Google Scholar] [CrossRef] [Green Version]
- Klamt, S.; Vogel, M.; Kapellen, T.; Hiemisch, A.; Prenzel, F.; Zachariae, S.; Ceglarek, U.; Thiery, J.; Kiess, W. Association between IgE-mediated allergies and diabetes mellitus type 1 in children and adolescents. Pediatr. Diabetes 2015, 16, 493–503. [Google Scholar] [CrossRef]
- Hsiao, Y.-T.; Cheng, W.-C.; Liao, W.-C.; Lin, C.-L.; Shen, T.-C.; Chen, W.-C.; Chen, C.-H.; Kao, C.-H. Type 1 Diabetes and Increased Risk of Subsequent Asthma: A Nationwide Population-Based Cohort Study. Medicine (Baltimore) 2015, 94, e1466. [Google Scholar] [CrossRef]
- Villa-Nova, H.; Spinola-Castro, A.M.; Garcia, F.E.; Solé, D. Prevalence of allergic diseases and/or allergic sensitisation in children and adolescents with type 1 diabetes mellitus. Allergol. Immunopathol. 2015, 43, 157–161. [Google Scholar] [CrossRef]
- Hörtenhuber, T.; Kiess, W.; Fröhlich-Reiterer, E.; Raile, K.; Stachow, R.; Bollow, E.; Rami-Merhar, B.; Holl, R.W. DPV-Wiss Study Group. Asthma in children and adolescents with type 1 diabetes in Germany and Austria: Frequency and metabolic control. Pediatr. Diabetes 2018, 19, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Metsala, J.; Lundqvist, A.; Virta, L.J.; Kaila, M.; Gissler, M.; Virtanen, S.M.; Nevalainen, J. The association between asthma and type 1 diabetes: A paediatric case-cohort study in Finland, years 1981–2009. Int. J. Epidemiol. 2018, 47, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Smeeth, L.; Hubbard, R. There is no evidence of an inverse relationship between TH2-mediated atopy and TH1-mediated autoimmune disorders: Lack of support for the hygiene hypothesis. J. Allergy Clin. Immunol. 2003, 111, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, C.R.; Shields, M.D.; Carson, D.J.; Patterson, C.C. A meta-analysis of the association between childhood type 1 diabetes and atopic disease. Diabetes Care 2003, 26, 2568–2574. [Google Scholar] [CrossRef] [Green Version]
- Cakir, M.; Akcay, S.; Karakas, T.; Gedik, Y.; Okten, A.; Orhan, F. Prevalence of atopy in children with type 1 diabetes mellitus, hepatitis B virus carriers, and healthy children: Role of T helper 1 (Th1)-type immune response. Allergy Asthma Proc. 2008, 29, 166–170. [Google Scholar] [CrossRef]
- Kainonen, E.; Rautava, S.; Korkeamäki, M.; Isolauri, E. Unique cytokine secretion profile in children with both type I diabetes and asthma distinct from that of solely diabetic or asthmatic children. Cytokine 2006, 34, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Rachmiel, M.; Bloch, O.; Shaul, A.A.; Ben-Yehudah, G.; Bistritzer, Z.; Weintrob, N.; Ofan, R.; Rapoport, M.J. Young patients with both type 1 diabetes mellitus and asthma have a unique IL-12 and IL-18 secretory pattern. Pediatr. Diabetes 2011, 12, 596–603. [Google Scholar] [CrossRef]
- Mcgeady, S. Immunocompetence and allergy. Pediatrics 2004, 113, 1107–1113. [Google Scholar]
- Gale, E.A. A missing link in the hygiene hypothesis? Diabetologia 2002, 45, 588–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stene, L.C.; Nafstad, P. Relation between occurrence of type 1 diabetes and asthma. Lancet 2011, 357, 607–608. [Google Scholar] [CrossRef]
- Kero, J.; Gissler, M.; Hemminki, E.; Isolauri, E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: A register study. J. Allergy Clin. Immunol. 2001, 108, 781–783. [Google Scholar] [CrossRef]
- Asher, M.; Montefort, S.; Björkstén, B.; Lai, C.K.W.; Strachan, D.P.; Weiland, S.K.; Williams, H.C.W.L. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Advani, A. Positioning time in range in diabetes management. Diabetologia 2020, 63, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.D. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2016, 375, 871–878. [Google Scholar] [CrossRef] [Green Version]

| Study Design | No. of T1D Patients (age, ys) | Spirometry | Plethysmography | DLCO | Other Findings | Correlation with Study Variables | |
|---|---|---|---|---|---|---|---|
| Buckingham et al. 1986 [36] | Observational without control group | 375 (7–25 ys) | <FVC | - | - | - | No significant relationship between decreased vital capacities and duration of diabetes |
| Primhak et al. 1987 [37] | Observational, Case-control | 88 (6–17 ys) | <FVC | - | - | - | No correlation between reduced FVC and duration of disease or mean HbA1c |
| Verrotti et al. 1993 [38] | Observational, without control group | 68 (6–22 ys) | Normal | Normal (>Raw) | - | Subgroup analysis: T1D with microalbuminuria vs. T1D without microalbuminuria | Microalbuminuria is not a risk factor for the development of abnormal pulmonary function. |
| Van Gent et al. 2002 [39] | Observational, without control group | 27 (age range NA, mean 12.8 ± 5 ys) | Normal | Normal (>Raw) | Normal | - | No correlation with age, disease duration or HbA1c |
| Cazzato et al. 2004 [40] | Observational, Case-control. 38% of cases were studied at onset of disease. | 38 (1–12 ys) | <FVC, <FEV1 | <RV >RV/TLC | < | Results corrected for pubertal stage All the alterations except < RV were also found in subgroup at onset of disease | <TLCO in female gender No correlation between pulmonary function indices and mean HbA1c, microalbuminuria, duration of disease |
| Villa et al. 2004 [41] | Observational, case-control | 39 (5–14 ys) | Normal | - | <DLCO/VA | Subgroup analysis: patient with good vs. poor metabolic control (Hba1c < 8% vs. ≥8%) | Predicted DLCO/VA% correlated with HbA1c levels |
| Mohamad et al. 2011 [42] | Observational, case-control | 60 (6–16 ys) | <FVC, <FEV1, <FEV1/FVC, <PEF | - | - | Subgroup analysis: patient with good vs. poor metabolic control (mean HbA1c < 8% vs. ≥8%) | EC and FEV1 were significantly lower in children with poor glycemic control No correlation with disease duration |
| Al-Saadi et al. 2011 [43] | Observational without control group | 52 (8–14 ys) | <FVC, <PEF, <MMEF | - | - | - | NA |
| Scaramuzza et al. 2012 [20] | Observational, case-control | 42, (10–20 ys) | <FVC, <FEV1 | <TLC | < | <DLCO Vc | <DLCO in presence of autonomic neuropathy at neurovegetative test (index alfa) No correlation between pulmonary function test and DLCO and HbA1c, microalbuminuria, BMI, disease duration |
| Pieniawska et al. 2012 [44] * | Observational, case-control | 35 (age range NA) | <VC >FEV1/FVC <PEF | - | - | - | No correlation between spirometric parameters and disease duration and HbA1c <VC related to high HbA1c |
| Martin-Frias et al. 2015 [21] | Observational, case-control | 100 (8–18 ys) | < FVC, <FEV1 >FEV1/FVC | >TLC e RV >RV/TLC (<Raw) | <DLCO <VA >DLCO/AV | Results corrected for pubertal stage Subgroup analysis: patient with good vs. poor metabolic control (mean Hba1c < 7.5 vs. ≥7.5%) | No differences in pulmonary function based on duration of disease or metabolic control <FEV1/FVC in poor metabolic control subgroup |
| Anik et al. 2020 [45] | Observational, case-control | 51 (3–15 ys) | <FEF 75 <FEF 25–75 | (>Raw) | - | - | Positive: Metabolic control at the time of the test (HbA1c), disease duration |
| Study Design | No. of T1D Patients (Age, ys) | Presence of Atopy | Allergic Test | Allergic Diseases | Asthma | Other Findings | |
|---|---|---|---|---|---|---|---|
| Tosca et al. 2009 [60] | Observational Case-control | 112 (7.8–16.9) | Sensitization to aeroallergenes similar to control group | SPT | No difference in the proportion of individuals with rhinits | No difference in the proportion of individuals with asthma | No T1D patients with “actual asthma” vs. 5, 8% in the control group (p = 0.009) |
| Jasser-Nitsche et al. 2017 [61] | Observational, Case-control | 104 (11.4 ± 4.4) | Allergen-specific sensitization similar to control group | RAST | No difference in the proportion of individuals with symptoms | ||
| Seiskari et al. 2018 [62] | Observational, Case-control | 147 in Finland (NA); 132 in Karelian | No significant difference in allergen specific sensitization in Finland, more frequent sensitization in Karelian patients | Total serum IgE RAST (birch, cat, egg albumin) | Karelian T1D patients reported more frequent allergic diseases | Karelian T1D patients reported more frequent asthma | |
| Black et al. 2011 [63] | Observational, without control group | 1683 (3–21) | Asthma was present in 10.0% of T1D youth | Youth with asthma had poor glycemic control | |||
| Klamt et al. 2015 [64] | Observational, Case-control | 94 (12.12 ± 4.55) | No significant difference in allergen specific sensitization | Total serum IgE RAST | T1D patients have more risk for allergic symptoms | No difference in personal history of asthma | |
| Hsiao et al. 2015 [65] | Observational, Case-control | 3545 (11.30 ± 5.04) | Incidence of asthma higher in T1D patients | HR of asthma higher in T1D patients hospitalized more than twice for diabetes. | |||
| Villa-Nova et al. 2015 [66] | Observational, without control group | 96 (4–18) | Positive SPT in 46.9% of T1D patients | SPT | Higher prevalence of allergic symptoms: rhinitis 52.1%, atopic eczema 9.4% | Prevalence of asthma: 59.1% | |
| Hortenuber et al. 2004 [67] | Prospective observational study | 51,926 patients (<20) | 1755 patients (3, 4%) had the diagnosis of asthma | In T1D patients with asthma: Higher insulin dosage Lower height-SDS Higher BMI-SDS | |||
| Metsala et al. 2018 [68] | Observational, Case-control | 8939 (5–14) | - | 602 with T1D and asthma | Previous diagnosis of asthma increased the risk of T1D; previous diagnosis of T1D decreased the risk of asthma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mameli, C.; Ghezzi, M.; Mari, A.; Cammi, G.; Macedoni, M.; Redaelli, F.C.; Calcaterra, V.; Zuccotti, G.; D’Auria, E. The Diabetic Lung: Insights into Pulmonary Changes in Children and Adolescents with Type 1 Diabetes. Metabolites 2021, 11, 69. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11020069
Mameli C, Ghezzi M, Mari A, Cammi G, Macedoni M, Redaelli FC, Calcaterra V, Zuccotti G, D’Auria E. The Diabetic Lung: Insights into Pulmonary Changes in Children and Adolescents with Type 1 Diabetes. Metabolites. 2021; 11(2):69. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11020069
Chicago/Turabian StyleMameli, Chiara, Michele Ghezzi, Alessandra Mari, Giulia Cammi, Maddalena Macedoni, Francesca Chiara Redaelli, Valeria Calcaterra, Gianvincenzo Zuccotti, and Enza D’Auria. 2021. "The Diabetic Lung: Insights into Pulmonary Changes in Children and Adolescents with Type 1 Diabetes" Metabolites 11, no. 2: 69. https://0-doi-org.brum.beds.ac.uk/10.3390/metabo11020069