Hybrid Polymer/Metal Oxide Thin Films for High Performance, Flexible Transistors
Abstract
:1. Introduction
2. Synthesis of Metal Oxides
3. Metal Oxide/Polymer Hybrid Films in Transistors
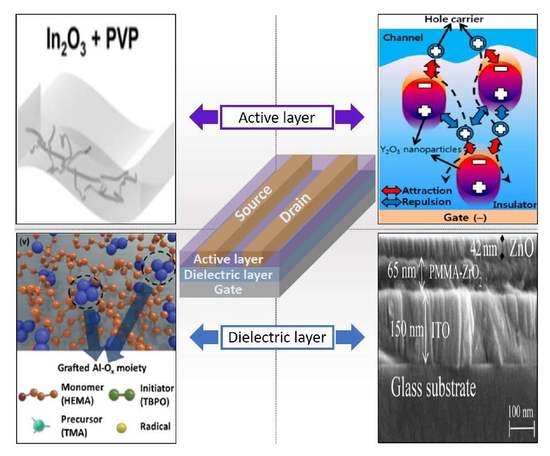
3.1. Active Channel Layers
3.2. Dielectric Layers
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ju, S.; Facchetti, A.; Xuan, Y.; Liu, J.; Ishikawa, F.; Ye, P.; Zhou, C.; Marks, T.J.; Janes, D.B. Fabrication of Fully Transparent Nanowire Transistors for Transparent and Flexible Electronics. Nat. Nanotechnol. 2007, 2, 378. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Vosguerichian, M.; Bao, Z. A Review of Fabrication and Applications of Carbon Nanotube Film-Based Flexible Electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- McCoul, D.; Hu, W.; Gao, M.; Mehta, V.; Pei, Q. Recent Advances in Stretchable and Transparent Electronic Materials. Adv. Electron. Mater. 2016, 2, 1500407. [Google Scholar] [CrossRef]
- Khang, D.-Y.; Jiang, H.; Huang, Y.; Rogers, J.A. A Stretchable Form of Single-Crystal Silicon for High-Performance Electronics on Rubber Substrates. Science 2006, 311, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.H.; Kim, Y.S.; Wu, J.; Liu, Z.; Song, J.; Kim, H.S.; Huang, Y.Y.; Hwang, K.C.; Rogers, J.A. Ultrathin Silicon Circuits with Strain-Isolation Layers and Mesh Layouts for High-Performance Electronics on Fabric, Vinyl, Leather, and Paper. Adv. Mater. 2009, 21, 3703–3707. [Google Scholar] [CrossRef]
- Faraji, S.; Danesh, E.; Tate, D.J.; Turner, M.L.; Majewski, L.A. Cyanoethyl Cellulose-Based Nanocomposite Dielectric for Low-Voltage, Solution-Processed Organic Field-Effect Transistors (OFETs). J. Phys. D 2016, 49, 185102. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, R.; Majewski, L.A.; Grell, M. High-Performance Organic Transistors Using Solution-Processed Nanoparticle-Filled High-k Polymer Gate Insulators. Adv. Mater. 2005, 17, 1535–1539. [Google Scholar] [CrossRef]
- Cai, W.; Wilson, J.; Zhang, J.; Park, S.; Majewski, L.; Song, A. Low-Voltage, Flexible InGaZnO Thin-Film Transistors Gated with Solution-Processed, Ultra-Thin Alx Oy. IEEE Electron Device Lett. 2018, 40, 36–39. [Google Scholar]
- Majewski, L.A.; Schroeder, R.; Grell, M. One Volt Organic Transistor. Adv. Mater. 2005, 17, 192–196. [Google Scholar] [CrossRef]
- Cai, W.; Park, S.; Zhang, J.; Wilson, J.; Li, Y.; Xin, Q.; Majewski, L.; Song, A. One-Volt IGZO Thin-Film Transistors With Ultra-Thin, Solution-Processed AlxOy Gate Dielectric. IEEE Electron Device Lett. 2018, 39, 375–378. [Google Scholar] [CrossRef]
- Cai, W.; Wilson, J.; Zhang, J.; Brownless, J.; Zhang, X.; Majewski, L.A.; Song, A. Significant Performance Enhancement of Very-Thin InGaZnO Thin-Film Transistors by a Self-Assembled Monolayer Treatment. ACS Appl. Electron. Mater. 2020, 2, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wong, C.P. Recent Advances in High-k Nanocomposite Materials for Embedded Capacitor Applications. IEEE. Trans. Dielectr. Electr. Insul. 2008, 15, 1322–1328. [Google Scholar]
- Liu, J.; Buchholz, D.B.; Hennek, J.W.; Chang, R.P.; Facchetti, A.; Marks, T.J. All-Amorphous-Oxide Transparent, Flexible Thin-Film Transistors. Efficacy of Bilayer Gate Dielectrics. J. Am. Chem. Soc. 2010, 132, 11934–11942. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Ju, B.-K.; Jang, J.; Yoon, Y.-S.; Kim, J.-K. High Mobility Organic Transistor Patterned by the Shadow-Mask with All Structure on a Plastic Substrate. J. Mater. Sci. 2007, 42, 1026–1030. [Google Scholar] [CrossRef]
- Lee, I.-Y.; Park, H.-Y.; Park, J.-H.; Yoo, G.; Lim, M.-H.; Park, J.; Rathi, S.; Jung, W.-S.; Kim, J.; Kim, S.-W. Poly-4-vinylphenol and Poly (melamine-co-formaldehyde)-Based Graphene Passivation Method for Flexible, Wearable and Transparent Electronics. Nanoscale 2014, 6, 3830–3836. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Tien, N.T.; Kim, D.; Jang, M.; Yoon, O.J.; Lee, N.E. A Flexible Reduced Graphene Oxide Field-Effect Transistor for Ultrasensitive Strain Sensing. Adv. Funct. Mater. 2014, 24, 117–124. [Google Scholar] [CrossRef]
- Schattka, J.H.; Shchukin, D.G.; Jia, J.; Antonietti, M.; Caruso, R.A. Photocatalytic Activities of Porous Titania and Titania/Zirconia Structures Formed by Using a Polymer Gel Templating Technique. Chem. Mater. 2002, 14, 5103–5108. [Google Scholar] [CrossRef]
- Kim, J.H.; Hwang, B.-U.; Kim, D.-I.; Kim, J.S.; Seol, Y.G.; Kim, T.W.; Lee, N.-E. Nanocomposites of Polyimide and Mixed Oxide Nanoparticles for High Performance Nanohybrid Gate Dielectrics in Flexible Thin Film Transistors. Electron. Mater. Lett. 2017, 13, 214–221. [Google Scholar] [CrossRef]
- Madusanka, N.; Shivareddy, S.G.; Hiralal, P.; Eddleston, M.D.; Choi, Y.; Oliver, R.A.; Amaratunga, G.A.J. Nanocomposites of TiO2/Cyanoethylated Cellulose with Ultra High Dielectric Constants. Nanotechnology 2016, 27, 195402. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, M.R.; Baral, J.K.; Hendricks, N.R.; Tang, Y.; Briseño, A.L.; Watkins, J.J. Solution Processable High Dielectric Constant Nanocomposites Based on ZrO2 Nanoparticles for Flexible Organic Transistors. ACS Appl. Mater. Interfaces 2013, 5, 13096–13103. [Google Scholar] [CrossRef]
- Yang, W.; Song, K.; Jung, Y.; Jeong, S.; Moon, J. Solution-Deposited Zr-doped AlOx Gate Dielectrics Enabling High-Performance Flexible Transparent Thin Film Transistors. J. Mater. Chem. C 2013, 1, 4275–4282. [Google Scholar] [CrossRef]
- Min, Y.-S.; Cho, Y.J.; Hwang, C.S. Atomic Layer Deposition of Al2O3 Thin Films from a 1-methoxy-2-methyl-2-propoxide Complex of Aluminum and Water. Chem. Mater. 2005, 17, 626–631. [Google Scholar] [CrossRef]
- Jeong, S.; Ha, Y.G.; Moon, J.; Facchetti, A.; Marks, T.J. Role of Gallium Doping in Dramatically Lowering Amorphous-Oxide Processing Temperatures for Solution-Derived Indium Zinc Oxide Thin-Film Transistors. Adv. Mater. 2010, 22, 1346–1350. [Google Scholar] [CrossRef] [PubMed]
- Hennek, J.W.; Smith, J.; Yan, A.; Kim, M.-G.; Zhao, W.; Dravid, V.P.; Facchetti, A.; Marks, T.J. Oxygen “getter” Effects on Microstructure and Carrier Transport in Low Temperature Combustion-Processed a-InXZnO (X = Ga, Sc, Y, La) Transistors. J. Am. Chem. Soc. 2013, 135, 10729–10741. [Google Scholar] [CrossRef] [PubMed]
- Banger, K.K.; Peterson, R.L.; Mori, K.; Yamashita, Y.; Leedham, T.; Sirringhaus, H. High Performance, Low Temperature Solution-Processed Barium and Strontium Doped Oxide Thin Film Transistors. Chem. Mater. 2014, 26, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.W.; Kim, J.; Kim, K.T.; Kang, J.G.; Kim, M.G.; Kim, K.H.; Ko, H.; Kim, Y.H.; Park, S.K. Highly Stable and Imperceptible Electronics Utilizing Photoactivated Heterogeneous Sol-Gel Metal–Oxide Dielectrics and Semiconductors. Adv. Mater. 2015, 27, 1182–1188. [Google Scholar] [CrossRef]
- Khanal, R.; Buchholz, D.B.; Chang, R.P.; Medvedeva, J.E. Composition-Dependent Structural and Transport Properties of Amorphous Transparent Conducting Oxides. Phys. Rev. B 2015, 91, 205203. [Google Scholar] [CrossRef] [Green Version]
- Nadaud, N.; Lequeux, N.; Nanot, M.; Jove, J.; Roisnel, T. Structural Studies of Tin-Doped Indium Oxide (ITO) and In4Sn3O12. J. Solid State Chem. 1998, 135, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.H.; Kang, S.-K.; Cho, I.-T.; Han, S.Y.; Chung, H.U.; Lee, D.J.; Shin, J.; Baek, G.W.; Kim, T.-I.; Lee, J.-H. Water-Soluble Thin Film Transistors and Circuits Based on Amorphous Indium–Gallium–Zinc Oxide. ACS Appl. Mater. Interfaces 2015, 7, 8268–8274. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, J.; Lee, S.-G.; Kim, Y.S. Water-Soluble Polymer Dielectric with Potential for High Performance Organic Thin-Film Transistors. Chem. Commun. 2010, 46, 3961–3963. [Google Scholar] [CrossRef]
- Byun, H.S.; Xu, Y.-X.; Song, C.K. Fabrication of High Performance Pentacene Thin Film Transistors Using Poly (4-vinylphenol) as the Gate Insulator on Polyethyleneterephthalate Substrates. Thin Solid Films 2005, 493, 278–281. [Google Scholar] [CrossRef]
- Kim, J.-M.; Lee, J.-W.; Kim, J.-K.; Ju, B.-K.; Kim, J.-S.; Lee, Y.-H.; Oh, M.-H. An Organic Thin-Film Transistor of High Mobility by Dielectric Surface Modification with Organic Molecule. Appl. Phys. Lett. 2004, 85, 6368–6370. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; You, E.; Hendricks, N.R.; Briseno, A.L.; Watkins, J.J. Flexible Low-Voltage Polymer Thin-Film Transistors Using Supercritical CO2-Deposited ZrO2 Dielectrics. ACS Appl. Mater. Interfaces 2012, 4, 2322–2324. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, F.; Cipolloni, S.; Mariucci, L.; Fortunato, G. High-Field-Effect-Mobility Pentacene Thin-Film Transistors with Polymethylmetacrylate Buffer Layer. Appl. Phys. Lett. 2005, 86, 203505. [Google Scholar] [CrossRef]
- Kato, T.; Suzuki, T.; Amamiya, T.; Irie, T.; Komiyama, M.; Yui, H. Effects of Macromolecules on the Crystallization of CaCO3 the Formation of Organic/Inorganic Composites. Supramol. Sci. 1998, 5, 411–415. [Google Scholar] [CrossRef]
- Zukas, B.G.; Gupta, N.R. Interphase Synthesis of Zinc Oxide Nanoparticles in a Droplet Flow Reactor. Ind. Eng. Chem. Res. 2017, 56, 7184–7191. [Google Scholar] [CrossRef]
- Ali, G.; Park, Y.J.; Kim, J.W.; Cho, S.O. A Green, General, and Ultrafast Route for the Synthesis of Diverse Metal Oxide Nanoparticles with Controllable Sizes and Enhanced Catalytic Activity. ACS Appl. Nano Mater. 2018, 1, 6112–6122. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, X.; Zheng, C.; Liu, Z. Two-Dimensional Porous Micro/Nano Metal Oxides Templated by Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 11984–11990. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous Sol–Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yu, X. Hydrothermal Synthesis and Photocatalytic Activity of Zinc Oxide Hollow Spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef]
- Ba, J.; Polleux, J.; Antonietti, M.; Niederberger, M. Non-Aqueous Synthesis of Tin Oxide Nanocrystals and Their Assembly into Ordered Porous Mesostructures. Adv. Mater. 2005, 17, 2509–2512. [Google Scholar] [CrossRef]
- Bilecka, I.; Djerdj, I.; Niederberger, M. One-Minute Synthesis of Crystalline Binary and Ternary Metal Oxide Nanoparticles. Chem. Commun. 2008, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Hu, X.; Yue, P.L.; Lu, G.Q.; Greenfield, P.F. Synthesis of Anatase TiO2 Supported on Porous Solids by Chemical Vapor Deposition. Catal. Today 2001, 68, 173–182. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, Y.; Li, J.; Gu, L.; Yuan, H.; Xiao, D. Synthesis of 3D-Nanonet Hollow Structured Co3O4 for High Capacity Supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Branco, P.S.; Parghi, K.; Shrikhande, J.J.; Pandey, R.K.; Ghumman, C.A.A.; Bundaleski, N.; Teodoro, O.M.N.D.; Jayaram, R.V. Synthesis and Characterization of Versatile MgO–ZrO2 Mixed Metal Oxide Nanoparticles and Their Applications. Catal. Sci. Technol. 2011, 1, 1653–1664. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ghose, R. Synthesis of Nanocrystalline CuO–ZnO Mixed Metal Oxide Powder by a Homogeneous Precipitation Method. Ceram. Int. 2014, 40, 10919–10926. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Cui, J.; Sun, J. Hydrothermal Synthesis of Self-Assembled Hierarchical Tungsten Oxides Hollow Spheres and Their Gas Sensing Properties. ACS Appl. Mater. Interfaces 2015, 7, 10108–10114. [Google Scholar] [CrossRef]
- Van Tong, P.; Hoa, N.D.; Van Duy, N.; Le, D.T.T.; Van Hieu, N. Enhancement of Gas-Sensing Characteristics of Hydrothermally Synthesized WO3 Nanorods by Surface Decoration with Pd Nanoparticles. Sens. Actuators B Chem. 2016, 223, 453–460. [Google Scholar] [CrossRef]
- Yeo, J.; Hong, S.; Kim, G.; Lee, H.; Suh, Y.D.; Park, I.; Grigoropoulos, C.P.; Ko, S.H. Laser-Induced Hydrothermal Growth of Heterogeneous Metal-Oxide Nanowire on Flexible Substrate by Laser Absorption Layer Design. ACS Nano 2015, 9, 6059–6068. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Wei, X. High-Performance, Room-Temperature, and No-Humidity-Impact Ammonia Sensor Based on Heterogeneous Nickel Oxide and Zinc Oxide Nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 3816–3824. [Google Scholar] [CrossRef]
- Lu, B.; Bai, J.; Bo, X.; Zhu, L.; Guo, L. A Simple Hydrothermal Synthesis of Nickel Hydroxide–Ordered Mesoporous Carbons Nanocomposites and Its Electrocatalytic Application. Electrochim. Acta 2010, 55, 8724–8730. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, M.C.; Ke, W.J.; Zheng, X.L.; Chen, Z.; Qin, P.L.; Xiong, L.B.; Lei, H.W.; Wan, J.W.; Wen, J. Enhanced Stability of Perovskite Solar Cells with Low-Temperature Hydrothermally Grown SnO2 Electron Transport Layers. Adv. Funct. Mater. 2016, 26, 6069–6075. [Google Scholar] [CrossRef]
- Ji, H.; Miao, X.; Wang, L.; Qian, B.; Yang, G. Microwave-Assisted Hydrothermal Synthesis of Sphere-like C/CuO and CuO Nanocrystals and Improved Performance as Anode Materials for Lithium-Ion Batteries. Powder Technol. 2013, 241, 43–48. [Google Scholar] [CrossRef]
- Yayapao, O.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. CTAB-Assisted Hydrothermal Synthesis of Tungsten Oxide Microflowers. J. Alloys Compd. 2011, 509, 2294–2299. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, L.; Chai, Z.-F.; Shi, W.-Q. Synthesis of ThO2 Nanostructures through a Hydrothermal Approach: Influence of Hexamethylenetetramine (HMTA) and Sodium Dodecyl Sulfate (SDS). RSC Adv. 2014, 4, 52209–52214. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, Z.; Guo, H.; Zhang, Y.; Zhang, Q.; Gan, L. A Facile PVP-Assisted Hydrothermal Fabrication of Fe2O3/Graphene Composite as High Performance Anode Material for Lithium Ion Batteries. J. Alloys Compd. 2013, 560, 208–214. [Google Scholar] [CrossRef]
- Vishwas, M.; Narasimha Rao, K.; Arjuna Gowda, K.V.; Chakradhar, R.P.S. Influence of Sn Doping on Structural, Optical and Electrical Properties of ZnO Thin Films Prepared by Cost Effective Sol–Gel Process. Spectrochim. Acta A 2012, 95, 423–426. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Mao, Y.; Xing, M.; Zhang, J. Preparation of Homogeneous Nitrogen-Doped Mesoporous TiO2 Spheres with Enhanced Visible-Light Photocatalysis. Appl. Catal. B Environ. 2015, 164, 352–359. [Google Scholar] [CrossRef]
- Soo, M.T.; Prastomo, N.; Matsuda, A.; Kawamura, G.; Muto, H.; Noor, A.F.M.; Lockman, Z.; Cheong, K.Y. Elaboration and Characterization of Sol–Gel Derived ZrO2 Thin Films Treated with Hot Water. Appl. Surf. Sci. 2012, 258, 5250–5258. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G.; Buha, J.; Polleux, J.; Ba, J.; Pinna, N. Nonaqueous Synthesis of Metal Oxide Nanoparticles:Review and Indium Oxide as Case Study for the Dependence of Particle Morphology on Precursors and Solvents. J. Sol-Gel Sci. Technol. 2006, 40, 259–266. [Google Scholar] [CrossRef]
- Athar, T.; Hakeem, A.; Ahmed, W. Synthesis of MgO Nanopowder via Non Aqueous Sol–gel Method. Adv. Sci. Lett. 2012, 7, 27–29. [Google Scholar] [CrossRef]
- Masthoff, I.C.; Kraken, M.; Menzel, D.; Litterst, F.J.; Garnweitner, G. Study of the Growth of Hydrophilic Iron Oxide Nanoparticles Obtained via the Non-Aqueous Sol–Gel Method. J. Sol-Gel Sci. Technol. 2016, 77, 553–564. [Google Scholar] [CrossRef]
- Singh, I.; Kumar, R.; Birajdar, B.I. Zirconium Doped TiO2 Nano-Powder via Halide Free Non-Aqueous Solvent Controlled Sol–gel Route. J. Environ. Chem. Eng. 2017, 5, 2955–2963. [Google Scholar] [CrossRef]
- Niederberger, M.; Bartl, M.H.; Stucky, G.D. Benzyl Alcohol and Transition Metal Chlorides as a Versatile Reaction System for the Nonaqueous and Low-Temperature Synthesis of Crystalline Nano-Objects with Controlled Dimensionality. J. Am. Chem. Soc. 2002, 124, 13642–13643. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Neri, G. Microwave-Assisted Synthesis of Metal Oxide Nanostructures for Gas Sensing Application: A Review. Sens. Actuators B Chem. 2016, 237, 749–775. [Google Scholar] [CrossRef]
- Xi, G.; He, Y.; Zhang, Q.; Xiao, H.; Wang, X.; Wang, C. Synthesis of Crystalline Microporous SnO2 via a Surfactant-Assisted Microwave Heating Method: A General and Rapid Method for the Synthesis of Metal Oxide Nanostructures. J. Phys. Chem. C 2008, 112, 11645–11649. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Supercapacitor Studies on NiO Nanoflakes Synthesized Through a Microwave Route. ACS Appl. Mater. Interfaces 2013, 5, 2188–2196. [Google Scholar] [CrossRef]
- Mondal, A.K.; Su, D.; Chen, S.; Kretschmer, K.; Xie, X.; Ahn, H.-J.; Wang, G. A Microwave Synthesis of Mesoporous NiCo2O4 Nanosheets as Electrode Materials for Lithium-Ion Batteries and Supercapacitors. ChemPhysChem 2015, 16, 169–175. [Google Scholar] [CrossRef]
- Ede, S.R.; Anantharaj, S.; Subramanian, B.; Rathishkumar, A.; Kundu, S. Microwave-Assisted Template-Free Synthesis of Ni3(BO3)2(NOB) Hierarchical Nanoflowers for Electrocatalytic Oxygen Evolution. Energy Fuels 2018, 32, 6224–6233. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave Chemistry for Inorganic Nanomaterials Synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-Temperature Fabrication of Transparent Flexible Thin-Film Transistors Using Amorphous Oxide Semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.B.; Ma, Q.; Alducin, D.; Ponce, A.; Jose-Yacaman, M.; Khanal, R.; Medvedeva, J.E.; Chang, R.P.H. The Structure and Properties of Amorphous Indium Oxide. Chem. Mater. 2014, 26, 5401–5411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.R.; Pattanasattayavong, P.; Anthopoulos, T.D. Solution-Processable Metal Oxide Semiconductors for Thin-Film Transistor Applications. Chem. Soc. Rev. 2013, 42, 6910–6923. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, D.; Li, M.; Xu, M.; Zou, J.; Tao, H.; Lan, L.; Wang, L.; Peng, J.; Cao, Y. A Flexible AMOLED Display on the PEN Substrate Driven by Oxide Thin-Film Transistors Using Anodized Aluminium Oxide as Dielectric. J. Mater. Chem. C 2014, 2, 1255–1259. [Google Scholar] [CrossRef]
- Cairns, D.R.; Witte, R.P.; Sparacin, D.K.; Sachsman, S.M.; Paine, D.C.; Crawford, G.P.; Newton, R.R. Strain-Dependent Electrical Resistance of Tin-Doped Indium Oxide on Polymer Substrates. Appl. Phys. Lett. 2000, 76, 1425–1427. [Google Scholar] [CrossRef]
- Peng, C.; Jia, Z.; Bianculli, D.; Li, T.; Lou, J. In Situ Electro-Mechanical Experiments and Mechanics Modeling of Tensile Cracking in Indium Tin Oxide Thin Films on Polyimide Substrates. J. Appl. Phys. 2011, 109, 103530. [Google Scholar] [CrossRef] [Green Version]
- Xing, G.Z.; Yi, J.B.; Yan, F.; Wu, T.; Li, S. Positive Magnetoresistance in Ferromagnetic Nd-Doped In2O3 Thin Films Grown by Pulse Laser Deposition. Appl. Phys. Lett. 2014, 104, 202411. [Google Scholar] [CrossRef] [Green Version]
- Imai, H.; Tominaga, A.; Hirashima, H.; Toki, M.; Asakuma, N. Ultraviolet-Reduced Reduction and Crystallization of Indium Oxide Films. J. Appl. Phys. 1999, 85, 203–207. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, K.; Coates, N.E.; Moses, D.; Nguyen, T.-Q.; Dante, M.; Heeger, A.J. Efficient Tandem Polymer Solar Cells Fabricated by All-Solution Processing. Science 2007, 317, 222–225. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Yang, C.; Gong, X.; Lee, K.; Heeger, A.J. Thermally Stable, Efficient Polymer Solar Cells with Nanoscale Control of the Interpenetrating Network Morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Kim, S.; Le, T.-H.; Kim, Y.; Park, G.; Park, C.S.; Kwon, O.S.; Yoon, H. Nanostructured Mesophase Electrode Materials: Modulating Charge-Storage Behavior by Thermal Treatment. Nanoscale 2017, 9, 17450–17458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beek, W.J.; Slooff, L.H.; Wienk, M.M.; Kroon, J.M.; Janssen, R.A. Hybrid Solar Cells Using a Zinc Oxide Precursor and a Conjugated Polymer. Adv. Funct. Mater. 2005, 15, 1703–1707. [Google Scholar] [CrossRef]
- Chen, C.-T.; Hsu, F.-C.; Kuan, S.-W.; Chen, Y.-F. The Effect of C60 on the ZnO-Nanorod Surface in Organic–Inorganic Hybrid Photovoltaics. Sol. Energy Mater. Sol. Cells 2011, 95, 740–744. [Google Scholar] [CrossRef]
- Benabid, F.; Kharchi, N.; Zouai, F.; Mourad, A.-H.I.; Benachour, D. Impact of Co-Mixing Technique and Surface Modification of ZnO Nanoparticles Using Stearic Acid on Their Dispersion into HDPE to Produce HDPE/ZnO Nanocomposites. Polym. Polym. Compos. 2019, 27, 389–399. [Google Scholar] [CrossRef]
- Vivekchand, S.; Kam, K.C.; Gundiah, G.; Govindaraj, A.; Cheetham, A.; Rao, C. Electrical Properties of Inorganic Nanowire–Polymer Composites. J. Mater. Chem. 2005, 15, 4922–4927. [Google Scholar] [CrossRef]
- Shim, M.; Javey, A.; Shi Kam, N.W.; Dai, H. Polymer Functionalization for Air-Stable n-Type Carbon Nanotube Field-Effect Transistors. J. Am. Chem. Soc. 2001, 123, 11512–11513. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Neal, A.T.; Si, M.; Ye, P.D. Molecular Doping of Multilayer MoS2 Field-Effect Transistors: Reduction in Sheet and Contact Resistances. IEEE Electron Device Lett. 2013, 34, 1328–1330. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Hong, W.; Thibau, E.S.; Aziz, H.; Lu, Z.-H.; Li, Y. Polyethylenimine (PEI) As an Effective Dopant To Conveniently Convert Ambipolar and p-Type Polymers into Unipolar n-Type Polymers. ACS Appl. Mater. Interfaces 2015, 7, 18662–18671. [Google Scholar] [CrossRef]
- Fabiano, S.; Braun, S.; Liu, X.; Weverberghs, E.; Gerbaux, P.; Fahlman, M.; Berggren, M.; Crispin, X. Poly(ethylene imine) Impurities Induce n-doping Reaction in Organic (Semi)Conductors. Adv. Mater. 2014, 26, 6000–6006. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Zeng, L.; Zhou, N.; Guo, P.; Shi, F.; Buchholz, D.B.; Ma, Q.; Yu, J.; Dravid, V.P.; Chang, R.P.H.; et al. Ultra-Flexible, “Invisible” Thin-Film Transistors Enabled by Amorphous Metal Oxide/Polymer Channel Layer Blends. Adv. Mater. 2015, 27, 2390–2399. [Google Scholar] [CrossRef]
- Jeong, E.G.; Kwon, J.H.; Kang, K.S.; Jeong, S.Y.; Choi, K.C. A Review of Highly Reliable Flexible Encapsulation Technologies towards Rollable and Foldable OLEDs. J. Inf. Disp. 2019, 19–32. [Google Scholar] [CrossRef]
- Huang, W.; Zeng, L.; Yu, X.; Guo, P.; Wang, B.; Ma, Q.; Chang, R.P.H.; Yu, J.; Bedzyk, M.J.; Marks, T.J.; et al. Metal Oxide Transistors via Polyethylenimine Doping of the Channel Layer: Interplay of Doping, Microstructure, and Charge Transport. Adv. Funct. Mater. 2016, 26, 6179–6187. [Google Scholar] [CrossRef]
- Huang, W.; Guo, P.; Zeng, L.; Li, R.; Wang, B.; Wang, G.; Zhang, X.; Chang, R.P.H.; Yu, J.; Bedzyk, M.J.; et al. Metal Composition and Polyethylenimine Doping Capacity Effects on Semiconducting Metal Oxide—Polymer Blend Charge Transport. J. Am. Chem. Soc. 2018, 140, 5457–5473. [Google Scholar] [CrossRef] [PubMed]
- Na, J.W.; Kim, H.J.; Hong, S.; Kim, H.J. Plasma Polymerization Enabled Polymer/Metal–Oxide Hybrid Semiconductors for Wearable Electronics. ACS Appl. Mater. Interfaces 2018, 10, 37207–37215. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Chen, C.; Zhang, J.; Wu, X.; Chen, H.; Guo, T. High Performance Inkjet-Printed Metal Oxide Thin Film Transistors via Addition of Insulating Polymer with Proper Molecular Weight. Appl. Phys. Lett. 2018, 112, 012102. [Google Scholar] [CrossRef]
- Ha, Y.-G.; Everaerts, K.; Hersam, M.C.; Marks, T.J. Hybrid Gate Dielectric Materials for Unconventional Electronic Circuitry. Acc. Chem. Res. 2014, 47, 1019–1028. [Google Scholar] [CrossRef]
- Wang, G.; Persson, N.; Chu, P.-H.; Kleinhenz, N.; Fu, B.; Chang, M.; Deb, N.; Mao, Y.; Wang, H.; Grover, M.A.; et al. Microfluidic Crystal Engineering of π-Conjugated Polymers. ACS Nano 2015, 9, 8220–8230. [Google Scholar] [CrossRef]
- Yeon Kwon, J.; Kyeong Jeong, J. Recent Progress in High Performance and Reliable N-type Transition Metal Oxide-Based Thin Film Transistors. Semicond. Sci. Technol. 2015, 30, 024002. [Google Scholar] [CrossRef]
- Wager, J.F.; Yeh, B.; Hoffman, R.L.; Keszler, D.A. An Amorphous Oxide Semiconductor Thin-Film Transistor Route to Oxide Electronics. Curr. Opin. Solid State Mater. Sci. 2014, 18, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Navan, R.R.; Prashanthi, K.; Shojaei Baghini, M.; Ramgopal Rao, V. Solution Processed Photopatternable High-k Nanocomposite Gate Dielectric for Low Voltage Organic Field Effect Transistors. Microelectron. Eng. 2012, 96, 92–95. [Google Scholar] [CrossRef]
- Lim, S.; Lee, K.H.; Kim, H.; Kim, S.H. Optimization of Nanocomposite Gate Insulators for Organic Thin Film Transistors. Org. Electron. 2015, 17, 144–150. [Google Scholar] [CrossRef]
- Hou, X.; Ng, S.C.; Zhang, J.; Chang, J.S. Polymer Nanocomposite Dielectric Based on P(VDF-TrFE)/PMMA/BaTiO3 for TIPs-Pentacene OFETs. Org. Electron. 2015, 17, 247–252. [Google Scholar] [CrossRef]
- Rasul, A.; Zhang, J.; Gamota, D.; Takoudis, C. High K Nanocomposite Dielectric for Printed Organic Electronics Applications. Microelectron. Eng. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Pecunia, V.; Banger, K.; Sirringhaus, H. High-Performance Solution-Processed Amorphous-Oxide-Semiconductor TFTs with Organic Polymeric Gate Dielectrics. Adv. Electron. Mater. 2015, 1, 1400024. [Google Scholar] [CrossRef] [Green Version]
- Sirringhaus, H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, H.; Zhao, Y.; Guo, Y.; Hu, W.; Yu, G.; Liu, Y. Substrate-Free Ultra-Flexible Organic Field-Effect Transistors and Five-Stage Ring Oscillators. Adv. Mater. 2013, 25, 5455–5460. [Google Scholar] [CrossRef]
- Khim, D.; Xu, Y.; Baeg, K.-J.; Kang, M.; Park, W.-T.; Lee, S.-H.; Kim, I.-B.; Kim, J.; Kim, D.-Y.; Liu, C.; et al. Large Enhancement of Carrier Transport in Solution-Processed Field-Effect Transistors by Fluorinated Dielectric Engineering. Adv. Mater. 2016, 28, 518–526. [Google Scholar] [CrossRef]
- Li, J.; Liu, D.; Miao, Q.; Yan, F. The Application of a high-k Polymer in Flexible Low-Voltage Organic Thin-Film Transistors. J. Mater. Chem. 2012, 22, 15998–16004. [Google Scholar] [CrossRef]
- Fukuda, K.; Sekine, T.; Shiwaku, R.; Morimoto, T.; Kumaki, D.; Tokito, S. Free-Standing Organic Transistors and Circuits with Sub-Micron Thicknesses. Sci. Rep. 2016, 6, 27450. [Google Scholar] [CrossRef]
- Yu, X.; Smith, J.; Zhou, N.; Zeng, L.; Guo, P.; Xia, Y.; Alvarez, A.; Aghion, S.; Lin, H.; Yu, J.; et al. Spray-Combustion Synthesis: Efficient Solution Route to High-Performance Oxide Transistors. Proc. Natl. Acad. Sci. USA 2015, 112, 3217–3222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Cao, Y.; Zhang, J.; Zhou, C. Large-Scale Complementary Macroelectronics Using Hybrid Integration of Carbon Nanotubes and IGZO Thin-Film Transistors. Nat. Commun. 2014, 5, 4097. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Zhang, J.; Gamota, D.; Singh, M.; Takoudis, C. Flexible High Capacitance Nanocomposite Gate Insulator for Printed Oorganic Field-Effect Transistors. Thin Solid Films 2010, 518, 7024–7028. [Google Scholar] [CrossRef]
- Yang, F.-Y.; Hsu, M.-Y.; Hwang, G.-W.; Chang, K.-J. High-Performance Poly(3-hexylthiophene) Top-Gate Transistors Incorporating TiO2 Nanocomposite Dielectrics. Org. Electron. 2010, 11, 81–88. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.W.; Kim, D.W.; Park, B.J.; Choi, H.J.; Choi, J.S. Pentacene Thin-Film Transistor with Poly(methyl methacrylate-co-methacrylic acid)/TiO2 Nanocomposite Gate Insulator. Thin Solid Films 2009, 518, 588–590. [Google Scholar] [CrossRef]
- Lee, W.-H.; Wang, C.C. Effect of Nanocomposite Gate Dielectric Rroughness on Pentacene Field-Effect Transistor. J. Vac. Sci. Technol. B 2009, 27, 1116–1121. [Google Scholar] [CrossRef]
- Lee, W.-H.; Wang, C.C.; Ho, J.C. Improved Performance of Pentacene Field-Effect Transistors Using a Nanocomposite Gate Dielectric. J. Vac. Sci. Technol. B 2009, 27, 601–605. [Google Scholar] [CrossRef]
- Noh, H.Y.; Seol, Y.G.; Kim, S.I.; Lee, N.E. Mechanically Flexible Low-Leakage Nanocomposite Gate Dielectrics for Flexible Organic Thin-Film Transistors. Electrochem. Solid State Lett. 2008, 11, H218–H221. [Google Scholar] [CrossRef]
- Kim, P.; Zhang, X.-H.; Domercq, B.; Jones, S.C.; Hotchkiss, P.J.; Marder, S.R.; Kippelen, B.; Perry, J.W. Solution-Processible High-Permittivity Nanocomposite Gate Insulators for Organic Field-Effect Transistors. Appl. Phys. Lett. 2008, 93, 013302. [Google Scholar] [CrossRef]
- Mohammadian, N.; Faraji, S.; Sagar, S.; Das, B.C.; Turner, M.L.; Majewski, L.A. One-Volt, Solution-Processed Organic Transistors with Self-Assembled Monolayer-Ta2O5 Gate Dielectrics. Materials 2019, 12, 2563. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Jun, T.; Kim, A.; Song, K.; Yeo, T.H.; Moon, J. Direct Photopatternable Organic–Inorganic Hybrid Gate Dielectric for Solution-Processed Flexible ZnO Thin Film Transistors. J. Mater. Chem. 2011, 21, 11879–11885. [Google Scholar] [CrossRef]
- Ha, Y.-G.; Jeong, S.; Wu, J.; Kim, M.-G.; Dravid, V.P.; Facchetti, A.; Marks, T.J. Flexible Low-Voltage Organic Thin-Film Transistors Enabled by Low-Temperature, Ambient Solution-Processable Inorganic/Organic Hybrid Gate Dielectrics. J. Am. Chem. Soc. 2010, 132, 17426–17434. [Google Scholar] [CrossRef]
- Morales-Acosta, M.D.; Quevedo-Lopez, M.A.; Gnade, B.E.; Ramírez-Bon, R.M. PMMA-SiO2 Organic–Inorganic Hybrid Films: Determination of Dielectric Characteristics. J. Sol-Gel Sci. Technol. 2011, 58, 218–224. [Google Scholar] [CrossRef]
- Morales-Acosta, M.D.; Quevedo-López, M.A.; Ramírez-Bon, R. PMMA–SiO2 Hybrid Films as Gate Dielectric for ZnO Based Thin-Film Transistors. Mater. Chem. Phys. 2014, 146, 380–388. [Google Scholar] [CrossRef]
- Morales-Acosta, M.D.; Alvarado-Beltrán, C.G.; Quevedo-López, M.A.; Gnade, B.E.; Mendoza-Galván, A.; Ramírez-Bon, R. Adjustable Structural, Optical and Dielectric Characteristics in Sol–Gel PMMA–SiO2 Hybrid Films. J. Non-Cryst. Solids 2013, 362, 124–135. [Google Scholar] [CrossRef]
- Alvarado-Beltrán, C.G.; Almaral-Sánchez, J.L.; Ramírez-Bon, R. Synthesis and Properties of PMMA-ZrO2 Organic–Inorganic Hybrid Films. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Lee, W.-J.; Park, W.-T.; Park, S.; Sung, S.; Noh, Y.-Y.; Yoon, M.-H. Large-Scale Precise Printing of Ultrathin Sol–Gel Oxide Dielectrics for Directly Patterned Solution-Processed Metal Oxide Transistor Arrays. Adv. Mater. 2015, 27, 5043–5048. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Thomas, S.R.; Faber, H.; Li, R.; McLachlan, M.A.; Patsalas, P.A.; Anthopoulos, T.D. Al-Doped ZnO Transistors Processed from Solution at 120 °C. Adv. Electron. Mater. 2016, 2, 1600070. [Google Scholar] [CrossRef]
- Alvarado-Beltrán, C.G.; Almaral-Sánchez, J.L.; Mejia, I.; Quevedo-López, M.A.; Ramirez-Bon, R. Sol–Gel PMMA–ZrO2 Hybrid Layers as Gate Dielectric for Low-Temperature ZnO-Based Thin-Film Transistors. ACS Omega 2017, 2, 6968–6974. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Ogomi, Y.; Chang, J.; Tsukamoto, S.; Kukihara, K.; Oshima, T.; Osada, N.; Yoshino, K.; Katayama, K.; Toyoda, T.; et al. Charge Transfer and Recombination at the Metal Oxide/CH3NH3PbClI2/Spiro-OMeTAD Interfaces: Uncovering the Detailed Mechanism Behind High Efficiency Solar Cells. Phys. Chem. Chem. Phys. 2014, 16, 19984–19992. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-H.; Zhang, X.; Piao, S.H.; Choi, H.J.; Bae, J.-H.; Park, J. Stability Study of Flexible 6,13-Bis(triisopropylsilylethynyl)pentacene Thin-Film Transistors with a Cross-Linked Poly(4-vinylphenol)/Yttrium Oxide Nanocomposite Gate Insulator. Polymers 2016, 8, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lim, S.H.; Kim, Y.S. Solution-Based TiO2−Polymer Composite Dielectric for Low Operating Voltage OTFTs. J. Am. Chem. Soc. 2010, 132, 14721–14723. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Lee, S.; Jeon, S.; Kwon, S.; Jeong, W.; Kim, H.; Shin, I.; Chang, H.J.; Park, H.-h.; Jeon, H. Al2O3 Buffer in a ZnO Thin Film Transistor with Poly-4-vinylphenol Dielectric. Semicond. Sci. Technol. 2008, 24, 025008. [Google Scholar] [CrossRef]
- Kim, M.J.; Pak, K.; Hwang, W.S.; Im, S.G.; Cho, B.J. Novel Vapor-Phase Synthesis of Flexible, Homogeneous Organic–Inorganic Hybrid Gate Dielectric with sub 5 nm Equivalent Oxide Thickness. ACS Appl. Mater. Interfaces 2018, 10, 37326–37334. [Google Scholar] [CrossRef]
- Son, B.-G.; Je, S.Y.; Kim, H.J.; Jeong, J.K. Modification of a Polymer Gate Insulator by Zirconium Oxide Doping for Low Temperature, High Performance Indium Zinc Oxide Transistors. RSC Adv. 2014, 4, 45742–45748. [Google Scholar] [CrossRef]
- Held, M.; Schießl, S.P.; Miehler, D.; Gannott, F.; Zaumseil, J. Polymer/Metal Oxide Hybrid Dielectrics for Low Voltage Field-Effect Transistors with Solution-Processed, High-Mobility Semiconductors. Appl. Phys. Lett. 2015, 107, 083301. [Google Scholar] [CrossRef]
- Ye, X.; Lin, H.; Yu, X.; Han, S.; Shang, M.; Zhang, L.; Jiang, Q.; Zhong, J. High Performance Low-Voltage Organic Field-Effect Transistors Enabled by Solution Processed Alumina and Polymer Bilayer Dielectrics. Synth. Met. 2015, 209, 337–342. [Google Scholar] [CrossRef]
- He, W.; Xu, W.; Peng, Q.; Liu, C.; Zhou, G.; Wu, S.; Zeng, M.; Zhang, Z.; Gao, J.; Gao, X.; et al. Surface Modification on Solution Processable ZrO2 High-k Dielectrics for Low Voltage Operations of Organic Thin Film Transistors. J. Phys. Chem. C 2016, 120, 9949–9957. [Google Scholar] [CrossRef]
- Ha, T.-J. Low-Voltage and Hysteresis-Free Organic Thin-Film Transistors Employing Solution-Processed Hybrid Bilayer Gate Dielectrics. Appl. Phys. Lett. 2014, 105, 043305. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.W.; Hwang, H.S.; Choi, D.; Ma, B.C.; Jung, J.; Chang, M. Hybrid Polymer/Metal Oxide Thin Films for High Performance, Flexible Transistors. Micromachines 2020, 11, 264. https://0-doi-org.brum.beds.ac.uk/10.3390/mi11030264
Jeong JW, Hwang HS, Choi D, Ma BC, Jung J, Chang M. Hybrid Polymer/Metal Oxide Thin Films for High Performance, Flexible Transistors. Micromachines. 2020; 11(3):264. https://0-doi-org.brum.beds.ac.uk/10.3390/mi11030264
Chicago/Turabian StyleJeong, Jae Won, Hye Suk Hwang, Dalsu Choi, Byung Chol Ma, Jaehan Jung, and Mincheol Chang. 2020. "Hybrid Polymer/Metal Oxide Thin Films for High Performance, Flexible Transistors" Micromachines 11, no. 3: 264. https://0-doi-org.brum.beds.ac.uk/10.3390/mi11030264