Dry Film Resist Laminated Microfluidic System for Electrical Impedance Measurements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
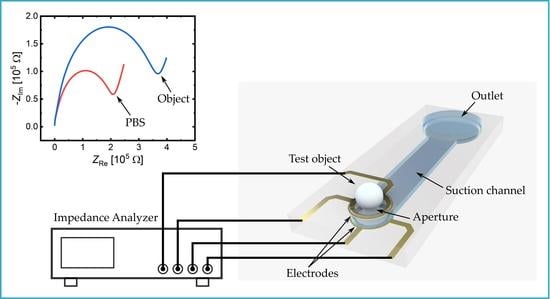
2.2. System Concept
2.3. Equivalent Electric Circuits
2.4. Microfluidic Chip Fabrication
3. Results
3.1. Measurement Setup
3.2. Electric Impedance Spectroscopy on Single Polystyrene Spheres
3.3. EIS Spectra and Fitting with Equivalent Electric Circuits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalkhal, F.; Chaney, K.H.; Muller, S.J. Optimization and application of dry film photoresist for rapid fabrication of high-aspect-ratio microfluidic devices. Microfluid. Nanofluid. 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Lorenz, H.; Paratte, L.; Luthier, R.; de Rooij, N.F.; Renaud, P. Low-cost technology for multilayer electroplated parts using laminated dry film resist. Sens. Actuators A Phys. 1996, 53, 364–368. [Google Scholar] [CrossRef]
- Heuschkel, M.O.; Guérin, L.; Buisson, B.; Bertrand, D.; Renaud, P. Buried microchannels in photopolymer for delivering of solutions to neurons in a network. Sens. Actuators B Chem. 1998, 48, 356–361. [Google Scholar] [CrossRef]
- Kukharenka, E.; Farooqui, M.M.; Grigore, L.; Kraft, M.; Hollinshead, N. Electroplating moulds using dry film thick negative photoresist. J. Micromech. Microeng. 2003, 13, S67–S74. [Google Scholar] [CrossRef] [Green Version]
- Vulto, P.; Huesgen, T.; Albrecht, B.; Urban, G.A. A full-wafer fabrication process for glass microfluidic chips with integrated electroplated electrodes by direct bonding of dry film resist. J. Micromech. Microeng. 2009, 19, 1–5. [Google Scholar] [CrossRef]
- Vulto, P.; Glade, N.; Altomare, L.; Bablet, J.; Tin, L.D.; Medoro, G.; Chartier, I.; Manaresi, N.; Tartagni, M.; Guerrieri, R. Microfluidic channel fabrication in dry film resist for production and prototyping of hybrid chips. Lab Chip 2005, 5, 158–162. [Google Scholar] [CrossRef]
- Paul, D.; Saias, L.; Pedinotti, J.C.; Chabert, M.; Magnifico, S.; Pallandre, A.; De Lambert, B.; Houdayer, C.; Brugg, B.; Peyrin, J.M.; et al. A “dry and wet hybrid” lithography technique for multilevel replication templates: Applications to microfluidic neuron culture and two-phase global mixing. Biomicrofluidics 2011, 5, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Mulloni, V.; Capuano, A.; Adami, A.; Quaranta, A.; Lorenzelli, L. A dry film technology for the manufacturing of 3-D multi-layered microstructures and buried channels for lab-on-chip. Microsyst. Technol. 2018, 25, 3219–3233. [Google Scholar] [CrossRef]
- Wangler, N.; Gutzweiler, L.; Kalkandjiev, K.; Müller, C.; Mayenfels, F.; Reinecke, H.; Zengerle, R.; Paust, N. High-resolution permanent photoresist laminate TMMF for sealed microfluidic structures in biological applications. J. Micromech. Microeng. 2011, 21, 1–9. [Google Scholar] [CrossRef]
- Guijt, R.M.; Candish, E.; Breadmore, M.C. Dry film microchips for miniaturised separations. Electrophoresis 2009, 30, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.; Greiner, C. SU-8: A photoresist for high-aspect-ratio and 3D submicron lithography. J. Micromech. Microeng. 2007, 17, R81–R95. [Google Scholar] [CrossRef] [Green Version]
- Gelorme, J.D.; Cox, R.J.; Gutierrez, S.A.R. Photoresist Composition and Printed Circuit Boards and Packages Made Therewith. U.S. Patent No. 4,882,245, 21 November 1989. [Google Scholar]
- Abgrall, P.; Lattes, C.; Conédéra, V.; Dollat, X.; Colin, S.; Gué, A.M. A novel fabrication method of flexible and monolithic 3D microfluidic structures using lamination of SU-8 films. J. Micromech. Microeng. 2006, 16, 113–121. [Google Scholar] [CrossRef]
- Meier, R.C.; Badilita, V.; Brunne, J.; Wallrabe, U.; Korvink, J.G. Complex three-dimensional high aspect ratio microfluidic network manufactured in combined PerMX dry-resist and SU-8 technology. Biomicrofluidics 2011, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Aljada, M.; Asthana, A. Fabrication of multilayer microstructures using dry film resist and deep reactive ion etcher. Micro Nano Lett. 2010, 5, 121–124. [Google Scholar] [CrossRef]
- Kalkandjiev, K.; Riegger, L.; Kosse, D.; Welsche, M.; Gutzweiler, L.; Zengerle, R.; Koltay, P. Microfluidics in silicon/polymer technology as a cost-efficient alternative to silicon/glass. J. Micromech. Microeng. 2011, 21, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Courson, R.; Cargou, S.; Conedera, V.; Fouet, M.; Blatche, M.C.; Serpentini, C.L.; Gue, A.M. Low-cost multilevel microchannel lab on chip: DF-1000 series dry film photoresist as a promising enabler. RSC Adv. 2014, 4, 54847–54853. [Google Scholar] [CrossRef]
- Abada, S.; Salvi, L.; Courson, R.; Daran, E.; Reig, B.; Doucet, J.B.; Camps, T.; Bardinal, V. Comparative study of soft thermal printing and lamination of dry thick photoresist films for the uniform fabrication of polymer MOEMS on small-sized samples. J. Micromech. Microeng. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Onoshima, D.; Wang, J.; Aki, M.; Arinaga, K.; Kaji, N.; Tokeshi, M.; Fujita, S.; Yokoyama, N.; Baba, Y. A deep microfluidic absorbance detection cell replicated from a thickly stacked SU-8 dry film resist mold. Anal. Methods 2012, 4, 4368–4372. [Google Scholar] [CrossRef]
- Stephan, K.; Pittet, P.; Renaud, L.; Kleimann, P.; Morin, P.; Ouaini, N.; Ferrigno, R. Fast prototyping using a dry film photoresist: Microfabrication of soft-lithography masters for microfluidic structures. J. Micromech. Microeng. 2007, 17, N69–N74. [Google Scholar] [CrossRef]
- Johnson, D.; Goettert, J.; Singh, V.; Yemane, D. SUEX Dry Film Resist—A New Material for High Aspect Ratio Lithography. 2011. Available online: https://www.lsu.edu/camd/files/DJ_AR2012_SUEXoverview.pdf (accessed on 26 March 2021).
- Johnson, D.; Goettert, J.; Singh, V. Opportunities for SUEX Dry Laminate Resist in Microfluidic MEMS Applications. 2012. Available online: https://www.lsu.edu/camd/files/DJ_AR2012_SUEX_fluidic.pdf (accessed on 26 March 2021).
- Xie, X.; Maharjan, S.; Liu, S.; Zhang, Y.S.; Livermore, C. A modular, reconfigurable microfabricated assembly platform for microfluidic transport and multitype cell culture and drug testing. Micromachines (Basel) 2019, 11, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, A.; Bernstein Toker, G.; Winter, S.; Cohen, S.S.; Ermak, O.; Peled, I.; Kotler, Z.; Gelbstein, Y. Hybrid structural electronics printing by novel dry film stereolithography and laser induced forward transfer. Nano Select 2021, 2, 979–991. [Google Scholar] [CrossRef]
- El Hasni, A.; Pfirrmann, S.; Kolander, A.; Yacoub-George, E.; König, M.; Landesberger, C.; Voigt, A.; Grützner, G.; Schnakenberg, U. Six-layer lamination of a new dry film negative-tone photoresist for fabricating complex 3D microfluidic devices. Microfluid. Nanofluid. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Hasni, A.E.; Schmitz, C.; Bui-Göbbels, K.; Bräunig, P.; Jahnen-Dechent, W.; Schnakenberg, U. Electrical impedance spectroscopy of single cells in hydrodynamic traps. Sens. Actuators B Chem. 2017, 248, 419–429. [Google Scholar] [CrossRef]
- Sun, T.; Bernabini, C.; Morgan, H. Single-colloidal particle impedance spectroscopy: Complete equivalent circuit analysis of polyelectrolyte microcapsules. Langmuir 2010, 26, 3821–3828. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.X.; Jiang, D.M.; Gu, C.L.; Qiu, Y.; Wan, H.; Wang, P. 3D microgroove electrical impedance sensing to examine 3D cell cultures for antineoplastic drug assessment. Microsyst. Nanoeng. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Chai, C.; Oh, S.W. Electrochemical impedimetric biosensors for food safety. Food Sci. Biotechnol. 2020, 29, 879–887. [Google Scholar] [CrossRef]
- Tang, W.L.; Tang, D.Z.; Ni, Z.H.; Xiang, N.; Yi, H. A portable single-cell analysis system integrating hydrodynamic trapping with broadband impedance spectroscopy. Sci. China Technol. Sci. 2017, 60, 1707–1715. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Rammelt, U.; Reinhard, G. On the applicability of a constant phase element (CPE) to the estimation of roughness of solid metal electrodes. Electrochim. Acta 1990, 35, 1045–1049. [Google Scholar] [CrossRef]
- Rossmacdonald, J. Note on the parameterization of the constant-phase admittance element. Solid State Ion. 1984, 13, 147–149. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhões, L.O.S. Impedance of constant phase element (CPE)-blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar] [CrossRef]
- Rasmussen, K.H.; Keller, S.S.; Jensen, F.; Jorgensen, A.M.; Hansen, O. SU-8 etching in inductively coupled oxygen plasma. Microelectron. Eng. 2013, 112, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Hong, G.; Holmes, A.S.; Heaton, M.E. SU8 resist plasma etching and its optimisation. Microsyst. Technol. 2004, 10, 357–359. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A. Variable Mott-Schottky plots acquisition by potentiodynamic electrochemical impedance spectroscopy. J. Solid State Electrochem. 2005, 9, 845–849. [Google Scholar] [CrossRef]
- Kummrow, A.; Theisen, J.; Frankowski, M.; Tuchscheerer, A.; Yildirim, H.; Brattke, K.; Schmidt, M.; Neukammer, J. Microfluidic structures for flow cytometric analysis of hydrodynamically focussed blood cells fabricated by ultraprecision micromachining. Lab Chip 2009, 9, 972–981. [Google Scholar] [CrossRef]
- Frankowski, M.; Theisen, J.; Kummrow, A.; Simon, P.; Ragusch, H.; Bock, N.; Schmidt, M.; Neukammer, J. Microflow cytometers with integrated hydrodynamic focusing. Sensors (Basel) 2013, 13, 4674–4693. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, M.; Yin, X. Three-dimensional (3D) hydrodynamic focusing for continuous sampling and analysis of adherent cells. Analyst 2011, 136, 3877–3883. [Google Scholar] [CrossRef]
- Paie, P.; Bragheri, F.; Vazquez, R.M.; Osellame, R. Straightforward 3D hydrodynamic focusing in femtosecond laser fabricated microfluidic channels. Lab Chip 2014, 14, 1826–1833. [Google Scholar] [CrossRef]
- Frankowski, M.; Simon, P.; Bock, N.; El-Hasni, A.; Schnakenberg, U.; Neukammer, J. Simultaneous optical and impedance analysis of single cells: A comparison of two microfluidic sensors with sheath flow focusing. Eng. Life Sci. 2015, 15, 286–296. [Google Scholar] [CrossRef]
- Qi, X.Y.; Yan, D.; Jiang, Z.; Cao, Y.K.; Yu, Z.Z.; Yavari, F.; Koratkar, N. Enhanced electrical conductivity in polystyrene nanocomposites at ultra-low graphene content. ACS Appl. Mater. Interfaces 2011, 3, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kilpatrick, P.K.; Melvin, E.; Velev, O.D. On-chip latex agglutination immunoassay readout by electrochemical impedance spectroscopy. Lab Chip 2012, 12, 4279–4286. [Google Scholar] [CrossRef] [PubMed]







| Parameter (Rel. Error). | RSol [Ω] | R [Ω] | C [F] |
|---|---|---|---|
| 1 mM PBS | 6.95 × 104 (1.67%) | 0.69 × 105 (1.85%) | 4.78 × 10−11 (2.18%) |
| 60 µm sphere | 10.5 × 104 (2.32%) | 3.69 × 105 (1.39%) | 1.86 × 10−11 (3.79%) |
| 80 µm sphere | 9.09 × 104 (2.91%) | 2.22 × 105 (1.63%) | 2.30 × 10−11 (3.21%) |
| 90 µm ZrO2 sphere | 9.08 × 104 (2.73%) | 2.34 × 105 (1.53%) | 2.22 × 10−11 (4.79%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Floehr, J.; Ingebrandt, S.; Schnakenberg, U. Dry Film Resist Laminated Microfluidic System for Electrical Impedance Measurements. Micromachines 2021, 12, 632. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12060632
Cao Y, Floehr J, Ingebrandt S, Schnakenberg U. Dry Film Resist Laminated Microfluidic System for Electrical Impedance Measurements. Micromachines. 2021; 12(6):632. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12060632
Chicago/Turabian StyleCao, Yuan, Julia Floehr, Sven Ingebrandt, and Uwe Schnakenberg. 2021. "Dry Film Resist Laminated Microfluidic System for Electrical Impedance Measurements" Micromachines 12, no. 6: 632. https://0-doi-org.brum.beds.ac.uk/10.3390/mi12060632