Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process
Abstract
:1. Introduction
2. Materials and Methods
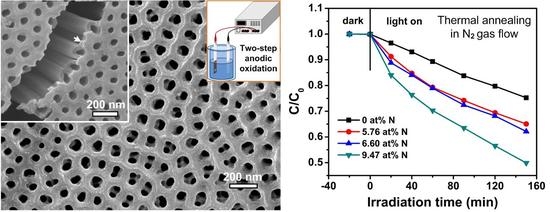
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roy, P.; Kim, D.; Lee, K.; Spiecker, E.; Schmuki, P. TiO2 nanotubes and their application in dye-sensitized solar cells. Nanoscale 2010, 2, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, B.; Musyaroah; Huda, I.; Indayani, W.; Seni Ramadhanti, S.; Endarko. The influence of various concentrations of N-doped TiO2 as photoanode to increase the efficiency of dye-sensitized solar cell. AIP Conf. Proc. 2017, 1788, 30128. [Google Scholar]
- Mor, G.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.K.; Huang, J.Y.; Zhang, H.F.; Subramaniam, V.P.; Tang, Y.X.; Gong, D.G.; Sundar, L.; Sun, L.; Chen, Z.; Lin, C.J. Nitrogen-doped TiO2 nanotube array films with enhanced photocatalytic activity under various light sources. J. Hazard. Mater. 2010, 184, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Hsu, H.-L.; Leu, J. TiO2 nanowires on anodic TiO2 nanotube arrays (TNWs/TNAs): Formation mechanism and photocatalytic performance. J. Electrochem. Soc. 2012, 159, H722–H727. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Enhanced photo-cleavage of water using titania nanotube arrays. Nano Lett. 2005, 5, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Nisar, J.; Topalian, Z.; De Sarkar, A.; Österlund, L.; Ahuja, R. TiO2-based gas sensor: A possible application to SO2. ACS Appl. Mater. Interfaces 2013, 5, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Sardroodi, J.J. A theoretical investigation of the interaction of Immucillin-A with N-doped TiO2 anatase nanoparticles: Applications to nanobiosensors and nanocarriers. Nanomed. Res. J. 2017, 2, 7–17. [Google Scholar]
- Roy, S.C.; Paulose, M.; Grimes, C.A. The effect of TiO2 nanotubes in the enhancement of blood clotting for the control of hemorrhage. Biomaterials 2007, 28, 4667–4672. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Zhang, P.; Fujitsuka, M.; Majima, T. TiO2 mesocrystal with nitrogen and fluorine codoping during topochemical transformation: Efficient visible light induced photocatalyst with the codopants. Appl. Catal. B Environ. 2016, 185, 181–188. [Google Scholar] [CrossRef]
- Preethi, L.K.; Antony, R.P.; Mathews, T.; Loo, S.C.J.; Wong, L.H.; Dash, S.; Tyagi, A.K. Nitrogen doped anatase-rutile heterostructured nanotubes for enhanced photocatalytic hydrogen production: Promising structure for sustainable fuel production. Int. J. Hydrogen Energy 2016, 41, 5865–5877. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Mazierski, P.; Nischk, M.; Gołkowska, M.; Lisowski, W.; Gazda, M.; Winiarski, M.J.; Klimczuk, T.; Zaleska-Medynska, A. Photocatalytic activity of nitrogen doped TiO2 nanotubes prepared by anodic oxidation: The effect of applied voltage, anodization time and amount of nitrogen dopant. Appl. Catal. B Environ. 2016, 196, 77–88. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- El Ruby Mohamed, A.; Rohani, S. Modified TiO2 nanotube arrays (TNTAs): Progressive strategies towards visible light responsive photoanode, a review. Energy Environ. Sci. 2011, 4, 1065–1086. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, G.; Chang, K.; Ling, Y.; Lin, Y.; Fitzmorris, B.C.; Liu, C.; Lu, X.; Tong, Y.; Zhang, J.Z.; et al. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett. 2013, 13, 3817–3823. [Google Scholar] [CrossRef] [PubMed]
- Gomathi Devi, L.; Kavitha, R. Review on modified N–TiO2 for green energy applications under UV/visible light: Selected results and reaction mechanisms. RSC Adv. 2014, 4, 28265–28299. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Cai, J.; Wu, Q.; Huang, P.; Su, Y.; Lin, C. N-doped TiO2 nanotube array photoelectrode for visible-light-induced photoelectrochemical and photoelectrocatalytic activities. Electrochim. Acta 2013, 108, 525–531. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.B.; Ogale, S.B. N-doped TiO2 nanoparticle based visible light photocatalyst by modi- fied peroxide sol-gel method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Preethi, L.K.; Antony, R.P.; Mathews, T.; Walczak, L.; Gopinath, C.S. A Study on Doped Heterojunctions in TiO2 Nanotubes: An Efficient Photocatalyst for Solar Water Splitting. Sci. Rep. 2017, 7, 14314. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, P.; Guo, L.; Chen, Z.; Wu, Q.; Ding, Y.; Zheng, W.; Cao, Y. The design of TiO2 nanostructures (nanoparticle, nanotube, and nanosheet) and their photocatalytic activity. J. Phys. Chem. C 2014, 118, 12727–12733. [Google Scholar] [CrossRef]
- Verma, R.; Gangwar, J.; Srivastava, A.K. Multiphase TiO2 nanostructures: A review of efficient synthesis, growth mechanism, probing capabilities, and applications in bio-safety and health. RSC Adv. 2017, 7, 44199–44224. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Park, J.; Kim, D.; Jeon, S. Monolithic 3D titania with ultrathin nanoshell structures for enhanced photocatalytic activity and recyclability. Nanoscale 2013, 5, 10384–10389. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Ahn, C.; Park, J.; Jeon, S. 3D nanostructured N-doped TiO2 photocatalysts with enhanced visible absorption. Nanoscale 2018, 10, 9747–9751. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, K.; Lai, Y. Fabrication, modification, and emerging applications of TiO2 nanotube arrays by electrochemical synthesis: A review. Int. J. Photoenergy 2013, 2013, 761971. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Guo, D.; Yu, L.; Zhang, W. Anodization fabrication of highly ordered TiO2 nanotubes. J. Phys. Chem. C 2009, 113, 12759–12765. [Google Scholar] [CrossRef]
- Kim, D.; Tsuchiya, H.; Fujimoto, S.; Schmidt-Stein, F.; Schmuki, P. Nitrogen-doped TiO2 mesosponge layers formed by anodization of nitrogen-containing Ti alloys. J. Solid State Electrochem. 2012, 16, 89–92. [Google Scholar] [CrossRef]
- Kim, D.; Fujimoto, S.; Schmuki, P.; Tsuchiya, H. Nitrogen doped anodic TiO2 nanotubes grown from nitrogen-containing Ti alloys. Electrochem. Commun. 2008, 10, 910–913. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, X.; Zhou, M.; Han, S.; Lei, L. Preparation of high efficient photoelectrode of N-F-codoped TiO2 nanotubes. J. Photochem. Photobiol. A Chem. 2008, 194, 152–160. [Google Scholar] [CrossRef]
- Kawai, T.; Kishimoto, Y.; Kifune, K. Photoluminescence studies of nitrogen-doped TiO2 powders prepared by annealing with urea. Philos. Mag. 2012, 92:33, 4088–4097. [Google Scholar] [CrossRef]
- Vitiello, R.P.; Macak, J.M.; Ghicov, A.; Tsuchiya, H.; Dick, L.F.P.; Schmuki, P. N-doping of anodic TiO2 nanotubes using heat treatment in ammonia. Electrochem. Commun. 2006, 8, 544–548. [Google Scholar] [CrossRef]
- Stranak, V.; Quaas, M.; Bogdanowicz, R.; Steffen, H.; Wulff, H.; Hubicka, Z.; Tichy, M.; Hippler, R. Effect of nitrogen doping on TiOxNy thin film formation at reactive high-power pulsed magnetron sputtering. J. Phys. D Appl. Phys. 2010, 43, 285203. [Google Scholar] [CrossRef]
- Stegemann, C.; Moraes, R.S.; Duarte, D.A.; Massi, M. Thermal annealing effect on nitrogen-doped TiO2 thin films grown by high power impulse magnetron sputtering plasma power source. Thin Solid Films 2017, 625, 49–55. [Google Scholar] [CrossRef]
- Vrakatseli, V.E.; Kalarakis, A.N.; Kalampounias, A.G.; Amanatides, E.K.; Mataras, D.S. Glancing Angle Deposition Effect on Structure and Light-Induced Wettability of RF-Sputtered TiO2 Thin Films. Micromachines 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Wang, D.; Yu, B.; Wang, C.; Zhou, F.; Liu, W. A novel protocol toward perfect alignment of anodized TiO2 nanotubes. Adv. Mater. 2009, 21, 1964–1967. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P. Optimization of photoelectrochemical water splitting performance on hierarchical TiO2 nanotube arrays. Energy Environ. Sci. 2012, 5, 6506–6512. [Google Scholar] [CrossRef]
- Pishkar, N.; Ghoranneviss, M.; Ghorannevis, Z.; Akbari, H. Study of the highly ordered TiO2 nanotubes physical properties prepared with two-step anodization. Results Phys. 2018, 9, 1246–1249. [Google Scholar] [CrossRef]
- Le, P.H.; Leu, J. Recent advances in TiO2 nanotube-based materials for photocatalytic applications designed by anodic oxidation. In Titanium Dioxide-Material for Sustainable Environment; Yang, D., Ed.; INTECH: London, UK, 2018; pp. 131–150. [Google Scholar]
- Sudhagar, P.; Asokan, K.; Ito, E.; Kang, Y.S. N-Ion-implanted TiO2 photoanodes in quantum dot-sensitized solar cells. Nanoscale 2012, 4, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.; Javornik, U.; Plavec, J.; Podgornik, A.; Rečnik, A. Self-assembly of multilevel branched rutile-type TiO2 structures via oriented lateral and twin attachment. Sci. Rep. 2016, 6, 24216. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, F. TiO2 nanotubes: Structure optimization for solar cells. J. Mater. Chem. 2011, 21, 9406–9418. [Google Scholar] [CrossRef]
- Agyeman, D.A.; Song, K.-S.; Kang, S.-H.; Jo, M.; Cho, E.; Kang, Y. An improved catalytic effect of nitrogen-doped TiO2 nanofibers for rechargeable Li–O2 batteries; the role of oxidation states and vacancies on the surface. J. Mater. Chem. A 2015, 3, 22557–22563. [Google Scholar] [CrossRef]
- Peng, F.; Cai, L.; Huang, L.; Yu, H.; Wang, H. Preparation of nitrogen-doped titanium dioxide with visible-light photocatalytic activity using a facile hydrothermal method. J. Phys. Chem. Solids 2008, 69, 1657–1664. [Google Scholar] [CrossRef]
- Sathish, M.; Viswanathan, B.; Viswanath, R.P.; Gopinath, C.S. Synthesis, characterization, electronic structure, and photocatalytic activity of nitrogen-doped TiO2 nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Kusumawardani, C.; Indiana, K. Synthesis of nanocrystalline N-doped TiO2 and its application on high efficiency of dye-sensitized solar cells. Sci. J. UBU 2010, 1, 1–8. [Google Scholar]
- Nikhil, A.; Anjusree, G.S.; Nair, S.V.; Nair, A.S. Visible light-induced photocatalytic activity of high surface area N-doped two-dimensional (2-D) TiO2 sheets. RSC Adv. 2015, 5, 88464–88470. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 026103. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.W.; Parkin, I.P. Nitrogen-doped TiO2 thin films: Photocatalytic applications for healthcare environments. Dalt. Trans. 2011, 40, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.; Giannini, C.; Cooper, J.K.; Loiudice, A.; Sharp, I.D.; Buonsanti, R. Substitutional or interstitial site selective nitrogen doping in TiO2 nanostructures. J. Phys. Chem. C 2015, 119, 7443–7452. [Google Scholar] [CrossRef]
- Tao, J.; Luttrell, T.; Batzill, M. A two-dimensional phase of TiO2 with a reduced bandgap. Nat. Chem. 2011, 3, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, G.; Xie, G.; Zhang, M.; Hou, Z.; He, Z. Gd3+, N-codoped trititanate nanotubes: Preparation, characterization and photocatalytic activity. Appl. Surf. Sci. 2011, 257, 3728–3732. [Google Scholar] [CrossRef]
- Jiang, H.; Yan, P.; Wang, Q.; Zang, S.; Li, J.; Wang, Q. High-performance Yb, N, P-tridoped anatase-TiO2 nano-photocatalyst with visible light response by sol-solvothermal method. Chem. Eng. J. 2013, 215–216, 348–357. [Google Scholar] [CrossRef]
- Preclíková, J.; Galář, P.; Trojánek, F.; Daniš, S.; Rezek, B.; Gregora, I.; Němcová, Y.; Malý, P. Nanocrystalline titanium dioxide films: Influence of ambient conditions on surface- and volume-related photoluminescence. J. Appl. Phys. 2010, 108, 113502. [Google Scholar] [CrossRef]
- Wang, E.; He, T.; Zhao, L.; Chen, Y.; Cao, Y. Improved visible light photocatalytic activity of titania doped with tin and nitrogen. J. Mater. Chem. 2011, 21, 144–150. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Sharma, S.D.; Saini, K.K.; Kant, C.; Sharma, C.P.; Jain, S.C. Photodegradation of dye pollutant under UV light by nanocatalyst doped titania thin films. Appl. Catal. B 2008, 84, 233–240. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Liu, R.; Gao, Z.; Yang, X.; Tu, Z.; Yang, F.; Ye, Z.; Cui, L.; Xu, C.; et al. Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J. Alloys Compd. 2016, 656, 24–32. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Liu, D.; Chi, L.; Hou, J. Ag and CdS nanoparticles co-sensitized TiO2 nanotubes for enhancing visible photoelectrochemical performance. Electrochim. Acta 2012, 83, 140–145. [Google Scholar] [CrossRef]






| N2 Gas Flow (cc/min) | N at % (Determined by XPS) | Reaction Rate (h−1) under Visible Light Irradiation |
|---|---|---|
| 0 | 0 | 1.15 × 10−1 |
| 200 | 5.76 | 1.66 × 10−1 |
| 500 | 6.60 | 1.79 × 10−1 |
| 350 | 9.47 | 2.59 × 10−1 |
| Photocatalyst | Synthesis Methods | Organic Pollutants | N Concentration (at. %) | Reaction Rate (h−1) | Ref. |
|---|---|---|---|---|---|
| Modified N-TNAs | Two-step anodization | Methylene blue | 9.47 | 0.26 | This study |
| N-TiO2 nanosheets | Chemical route | Methyl orange | - | 0.45 | [52] |
| N-TNAs | Anodic oxidation | RhB dye | - | 0.11 | [22] |
| N-TNAs | Anodic oxidation | Methyl orange | - | 1.62 | [4] |
| N-TiO2 nanowires | Hydrothermal method | Methylene blue | - | 0.13 | [63] |
| TiO2 nanoparticles | Sol-gel | Methylene blue | 5–10 | - | [23] |
| CdS–Ag/TiO2 NTs | Two-step anodization | Methyl orange | - | 0.13 | [64] |
| N-TiO2 nanoparticles | Sol-solvothermal process | Methylene blue | - | 0.44 | [58] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.H.; Hieu, L.T.; Lam, T.-N.; Hang, N.T.N.; Truong, N.V.; Tuyen, L.T.C.; Phong, P.T.; Leu, J. Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process. Micromachines 2018, 9, 618. https://0-doi-org.brum.beds.ac.uk/10.3390/mi9120618
Le PH, Hieu LT, Lam T-N, Hang NTN, Truong NV, Tuyen LTC, Phong PT, Leu J. Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process. Micromachines. 2018; 9(12):618. https://0-doi-org.brum.beds.ac.uk/10.3390/mi9120618
Chicago/Turabian StyleLe, Phuoc Huu, Le Trung Hieu, Tu-Ngoc Lam, Nguyen Thi Nhat Hang, Nguyen Van Truong, Le Thi Cam Tuyen, Pham Thanh Phong, and Jihperng Leu. 2018. "Enhanced Photocatalytic Performance of Nitrogen-Doped TiO2 Nanotube Arrays Using a Simple Annealing Process" Micromachines 9, no. 12: 618. https://0-doi-org.brum.beds.ac.uk/10.3390/mi9120618