Hypergolic Ignition of 1,3-Cyclodienes by Fuming Nitric Acid toward the Fast and Spontaneous Formation of Carbon Nanosheets at Ambient Conditions
Abstract
:1. Introduction
2. Materials and Methods
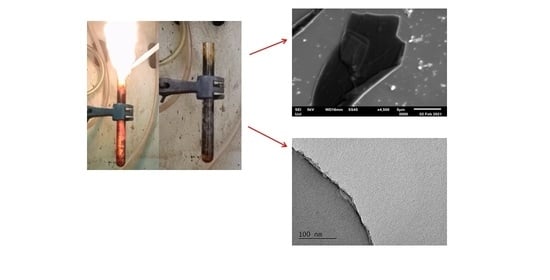
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A. The era of carbon allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Shen, W. Carbon Nanosheets: Synthesis and Application. ChemSusChem 2015, 8, 2004–2027. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhuang, X.; Lei, C.; Lei, L.; Hou, Y.; Mai, Y.; Feng, X. Porous carbon nanosheets: Synthetic strategies and electrochemical energy related applications. Nano Today 2019, 24, 103–119. [Google Scholar] [CrossRef]
- Schneider, S.; Hawkins, T.; Rosander, M.; Vaghjiani, G.; Chambreau, S.; Drake, G. Ionic Liquids as Hypergolic Fuels. Energy Fuels 2008, 22, 2871–2872. [Google Scholar] [CrossRef]
- Yuan, W.-L.; Zhang, L.; Tao, G.-H.; Wang, S.-L.; Wang, Y.; Zhu, Q.-H.; Zhang, G.-H.; Zhang, Z.; Xue, Y.; Qin, S.; et al. Designing high-performance hypergolic propellants based on materials genome. Sci. Adv. 2020, 6, eabb1899. [Google Scholar] [CrossRef]
- Elzein, B.; Jobin, O.; Robert, E. Reducing the Ignition Delay of Hypergolic Hybrid Rocket Fuels. J. Propuls. Power 2021, 37, 77–85. [Google Scholar] [CrossRef]
- Pfeil, M.A.; Dennis, J.D.; Son, S.F.; Heister, S.D.; Pourpoint, T.L.; Ramachandran, P.V. Characterization of Ethylenediamine Bisborane as a Hypergolic Hybrid Rocket Fuel Additive. J. Propuls. Power 2015, 31, 365–372. [Google Scholar] [CrossRef]
- Baikousi, M.; Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Avgeropoulos, A.; Gournis, D.; Karakassides, M.A. Direct production of carbon nanosheets by self-ignition of pyrophoric lithium dialkylamides in air. Mater. Lett. 2019, 254, 58–61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Spyrou, K.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Synthesis of Highly Crystalline Graphite from Spontaneous Ignition of In Situ Derived Acetylene and Chlorine at Ambient Conditions. Molecules 2020, 25, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalmpes, N.; Asimakopoulos, G.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Functional Carbon Materials Derived through Hypergolic Reactions at Ambient Conditions. Nanomaterials 2020, 10, 566. [Google Scholar] [CrossRef] [Green Version]
- Chalmpes, N.; Spyrou, K.; Vasilopoulos, K.C.; Bourlinos, A.B.; Moschovas, D.; Avgeropoulos, A.; Gioti, C.; Karakassides, M.A.; Gournis, D. Hypergolics in Carbon Nanomaterials Synthesis: New Paradigms and Perspectives. Molecules 2020, 25, 2207. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Tantis, I.; Bakandritsos, A.; Bourlinos, A.B.; Karakassides, M.A.; Gournis, D. Rapid Carbon Formation from Spontaneous Reaction of Ferrocene and Liquid Bromine at Ambient Conditions. Nanomaterials 2020, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Bourlinos, A.B.; Šedajová, V.; Kupka, V.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic Materials Synthesis through Reaction of Fuming Nitric Acid with Certain Cyclopentadienyl Compounds. C—J. Carbon Res. 2020, 6, 61. [Google Scholar] [CrossRef]
- Chalmpes, N.; Bourlinos, A.B.; Talande, S.; Bakandritsos, A.; Moschovas, D.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Nanocarbon from Rocket Fuel Waste: The Case of Furfuryl Alcohol-Fuming Nitric Acid Hypergolic Pair. Nanomaterials 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Chalmpes, N.; Moschovas, D.; Tantis, I.; Bourlinos, A.B.; Bakandritsos, A.; Fotiadou, R.; Patila, M.; Stamatis, H.; Avgeropoulos, A.; Karakassides, M.A.; et al. Carbon Nanostructures Derived through Hypergolic Reaction of Conductive Polymers with Fuming Nitric Acid at Ambient Conditions. Molecules 2021, 26, 1595. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.-S. Structural Study of the Activated Carbon Fiber using Laser Raman Spectroscopy. Carbon Lett. 2008, 9, 127–130. [Google Scholar] [CrossRef]
- Tsirka, K.; Katsiki, A.; Chalmpes, N.; Gournis, D.; Paipetis, A.S. Mapping of Graphene Oxide and Single Layer Graphene Flakes—Defects Annealing and Healing. Front. Mater. 2018, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Bourlinos, A.B.; Giannelis, E.P.; Sanakis, Y.; Bakandritsos, A.; Karakassides, M.; Gjoka, M.; Petridis, D. A graphite oxide-like carbogenic material derived from a molecular precursor. Carbon 2006, 44, 1906–1912. [Google Scholar] [CrossRef]
- Kouloumpis, A.; Thomou, E.; Chalmpes, N.; Dimos, K.; Spyrou, K.; Bourlinos, A.B.; Koutselas, I.; Gournis, D.; Rudolf, P. Graphene/Carbon Dot Hybrid Thin Films Prepared by a Modified Langmuir–Schaefer Method. ACS Omega 2017, 2, 2090–2099. [Google Scholar] [CrossRef] [Green Version]
- Chalmpes, N.; Kouloumpis, A.; Zygouri, P.; Karouta, N.; Spyrou, K.; Stathi, P.; Tsoufis, T.; Georgakilas, V.; Gournis, D.; Rudolf, P. Layer-by-Layer Assembly of Clay–Carbon Nanotube Hybrid Superstructures. ACS Omega 2019, 4, 18100–18107. [Google Scholar] [CrossRef] [PubMed]
- Potsi, G.; Bourlinos, A.B.; Mouselimis, V.; Poláková, K.; Chalmpes, N.; Gournis, D.; Kalytchuk, S.; Tomanec, O.; Błoński, P.; Medveď, M.; et al. Intrinsic photoluminescence of amine-functionalized graphene derivatives for bioimaging applications. Appl. Mater. Today 2019, 17, 112–122. [Google Scholar] [CrossRef]
- Rommozzi, E.; Zannotti, M.; Giovannetti, R.; D’Amato, C.A.; Ferraro, S.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Reduced Graphene Oxide/TiO2 Nanocomposite: From Synthesis to Characterization for Efficient Visible Light Photocatalytic Applications. Catalysts 2018, 8, 598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, N.; Yang, C.; Wei, H.; Yang, Z.; Wang, Y.; Wei, L.; Zhao, J.; Xu, Z.J.; Zhang, Y. Free-standing functional graphene reinforced carbon films with excellent mechanical properties and superhydrophobic characteristic. Compos. Part A Appl. Sci. Manuf. 2015, 74, 96–106. [Google Scholar] [CrossRef]
- Spyrou, K.; Calvaresi, M.; Diamanti, E.K.; Tsoufis, T.; Gournis, D.; Rudolf, P.; Zerbetto, F. Graphite Oxide and Aromatic Amines: Size Matters. Adv. Funct. Mater. 2015, 25, 263–269. [Google Scholar] [CrossRef]
- Grzyb, B.; Gryglewicz, S.; Śliwak, A.; Díez, N.; Machnikowski, J.; Gryglewicz, G. Guanidine, amitrole and imidazole as nitrogen dopants for the synthesis of N-graphenes. RSC Adv. 2016, 6, 15782–15787. [Google Scholar] [CrossRef]
- Tsoukatos, T.; Avgeropoulos, A.; Hadjichristidis, N.; Hong, K.; Mays, J.W. Model Linear Block Co-, Ter-, and Quaterpolymers of 1,3-Cyclohexadiene with Styrene, Isoprene, and Butadiene. Macromolecules 2002, 35, 7928–7935. [Google Scholar] [CrossRef]
- Misichronis, K.; Rangou, S.; Avgeropoulos, A. Synthesis and Molecular and Morphological Characterization of Poly(p-Trimethylsilyl Styrene) and Diblock Copolymers with Poly(1,3-Cyclohexadiene). Int. J. Polym. Anal. Charact. 2008, 13, 136–148. [Google Scholar] [CrossRef]
- Misichronis, K.; Rangou, S.; Ashcraft, E.; Kumar, R.; Dadmun, M.; Sumpter, B.G.; Zafeiropoulos, N.E.; Mays, J.W.; Avgeropoulos, A. Synthesis, characterization (molecular–morphological) and theoretical morphology predictions of linear triblock terpolymers containing poly(cyclohexadiene). Polymer 2013, 54, 1480–1489. [Google Scholar] [CrossRef] [Green Version]
- Misichronis, K.; Chen, J.; Kahk, J.K.; Imel, A.; Dadmun, M.; Hong, K.; Hadjichristidis, N.; Mays, J.W.; Avgeropoulos, A. Diblock copolymers of polystyrene-b-poly(1,3-cyclohexadiene) exhibiting unique three-phase microdomain morphologies. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1564–1572. [Google Scholar] [CrossRef]
- Misichronis, K.; Chen, J.; Imel, A.; Kumar, R.; Thostenson, J.; Hong, K.; Dadmun, M.; Sumpter, B.G.; Kennemur, J.G.; Hadjichristidis, N.; et al. Investigations on the Phase Diagram and Interaction Parameter of Poly(styrene-b-1,3-cyclohexadiene) Copolymers. Macromolecules 2017, 50, 2354–2363. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalmpes, N.; Moschovas, D.; Bourlinos, A.B.; Spyrou, K.; Vasilopoulos, K.C.; Avgeropoulos, A.; Karakassides, M.A.; Gournis, D. Hypergolic Ignition of 1,3-Cyclodienes by Fuming Nitric Acid toward the Fast and Spontaneous Formation of Carbon Nanosheets at Ambient Conditions. Micro 2021, 1, 15-27. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010003
Chalmpes N, Moschovas D, Bourlinos AB, Spyrou K, Vasilopoulos KC, Avgeropoulos A, Karakassides MA, Gournis D. Hypergolic Ignition of 1,3-Cyclodienes by Fuming Nitric Acid toward the Fast and Spontaneous Formation of Carbon Nanosheets at Ambient Conditions. Micro. 2021; 1(1):15-27. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010003
Chicago/Turabian StyleChalmpes, Nikolaos, Dimitrios Moschovas, Athanasios B. Bourlinos, Konstantinos Spyrou, Konstantinos C. Vasilopoulos, Apostolos Avgeropoulos, Michael A. Karakassides, and Dimitrios Gournis. 2021. "Hypergolic Ignition of 1,3-Cyclodienes by Fuming Nitric Acid toward the Fast and Spontaneous Formation of Carbon Nanosheets at Ambient Conditions" Micro 1, no. 1: 15-27. https://0-doi-org.brum.beds.ac.uk/10.3390/micro1010003