Metagenomic Sequencing Identifies Highly Diverse Assemblages of Dinoflagellate Cysts in Sediments from Ships’ Ballast Tanks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Samples
2.2. High-Throughput Metagenomic Approach
2.2.1. Primers Design
2.2.2. DNA Extraction, PCR Amplification, Pyrosequencing, and Data Processing
2.2.3. Data Analyses
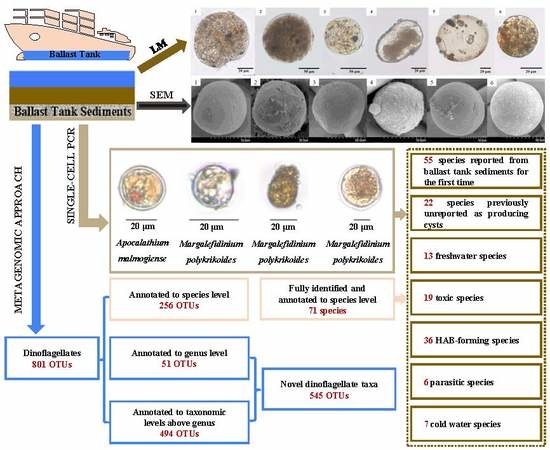
2.3. LM and SEM Observations of Dinoflagellate Cysts
2.4. Single-Cell PCR, Cloning and Sequencing
3. Results
3.1. Salinity and Temperature of the Samples
3.2. Results of High-Throughput Metagenomic Sequencing
3.2.1. General Descriptions
3.2.2. Diversity of Dinoflagellate Taxa
3.2.3. Most Abundant and Rarest Taxa
3.2.4. Habitat Assignments
3.2.5. Harmful and Parasitic Forms
3.2.6. Species Previously Unreported to Produce Cysts
3.3. Similarity and Difference in Species Composition Among Samples
3.4. LM and SEM Micrographs of Cysts
3.5. Dinoflagellate Cysts Identified Via Single-Cell PCR Sequencing
4. Discussion
4.1. Dinoflagellates in Ships’ Ballast Tanks—Comparison with Previous Studies
4.2. Both Marine and Freshwater Dinoflagellate Species Found
4.3. Potentially Harmful and/or Toxic Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bolch, C.J.S.; de Salas, M.F. A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australasia. Harmful Algae 2007, 6, 465–485. [Google Scholar] [CrossRef]
- Fowler, N.; Tomas, C.; Baden, D.; Campbell, L.; Bourdelais, A. Chemical analysis of Karenia papilionacea. Toxicon 2015, 101, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M.; Bolch, C.J. Transport of toxic dinoflagellate cysts via ships’ ballast water. Mar. Pollut. Bull. 1991, 22, 27–30. [Google Scholar] [CrossRef]
- Lohan, K.M.P.; Fleischer, R.C.; Carney, K.J.; Holzer, K.K.; Ruiz, G.M. Molecular characterisation of protistan species and communities in ships’ ballast water across three US coasts. Divers. Distrib. 2017, 23, 680–691. [Google Scholar] [CrossRef]
- Smayda, T.J. Reflections on the ballast water dispersal−harmful algal bloom paradigm. Harmful Algae 2007, 6, 601–622. [Google Scholar] [CrossRef]
- Steichen, J.L.; Schulze, A.; Brinkmeyer, R.; Quigg, A. All aboard! A biological survey of ballast water onboard vessels spanning the North Atlantic Ocean. Mar. Pollut. Bull. 2014, 87, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarska, I.; Ehrman, J.M. High colonization and propagule pressure by ship ballast as a vector for the diatom genus Pseudo-nitzschia. Manag. Biol. Invasions 2015, 6, 31–43. [Google Scholar] [CrossRef]
- Ostenfeld, C.H. On the immigration of Biddulphia sinensis Grev, and its occurrence in the North Sea during 1903-1907 and on its use for the study of the direction and rate of flow of the currents. Medd. Fra Komm. Dan. Fisk-Og Havundersøgelser: Ser. Plankton 1908, 1, 1–44. [Google Scholar]
- Carlton, J.T. Transoceanic and interoceanic dispersal of coastal marine organisms: The biology of ballast water. Oceanogr. Mar. Biol. 1985, 23, 313–371. [Google Scholar]
- Hallegraeff, G.M.; Bolch, C.J. Transport of diatom and dinoflagellate resting spores in ships’ ballast water: Implications for plankton biogeography and aquaculture. J. Plankton Res. 1992, 14, 1067–1084. [Google Scholar] [CrossRef]
- McCarthy, H.P.; Crowder, L.B. An overlooked scale of global transport: Phytoplankton species richness in ships’ ballast water. Biol. Invasions 2000, 2, 321–322. [Google Scholar] [CrossRef]
- Carlton, J.T.; Geller, J.B. Ecological roulette: The global transport of nonindigenous marine organisms. Science 1993, 261, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Scalco, E.; Audic, S.; Vincenta, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; de Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, E.M. Dinoflagellate resting cysts and ballast water discharges in Scottish ports. In Ballast Water: Ecological and Fisheries Implications/ICES Conference (83rd Statutory Meeting), 21–29 September 1995, Aalborg, Denmark; Carlton, J.T., Ed.; ICES: Aalborg, Denmark, 1995. [Google Scholar]
- Hamer, J.P.; Lucas, I.A.N.; McCollin, T.A. Harmful dinoflagellate resting cysts in ships’ ballast tank sediments: Potential for introduction into English and Welsh waters. Phycologia 2001, 40, 246–255. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Hallegraeff, G.M.; Melia, G.; Cohen, A.; Bowers, H.A.; Oldach, D.W.; Parrow, M.W.; Sullivan, M.J.; Zimba, P.V.; Allen, E.H.; et al. Phytoplankton and bacterial assemblages in ballast water of US military ships as a function of port of origin, voyage time, and ocean exchange practices. Harmful Algae 2007, 6, 486–518. [Google Scholar] [CrossRef]
- Casas-Monroy, O.; Roy, S.; Rochon, A. Dinoflagellate cysts in ballast sediments: Differences between Canada’s east coast, west coast and the Great Lakes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 254–276. [Google Scholar] [CrossRef]
- Gómez, F.; Richlen, M.L.; Anderson, D.M. Molecular characterization and morphology of Cochlodinium strangulatum, the type species of Cochlodinium, and Margalefidinium gen. nov. for C. polykrikoides and allied species (Gymnodiniales, Dinophyceae). Harmful Algae 2017, 63, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Gobler, C.J. Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae 2009, 8, 454–462. [Google Scholar] [CrossRef]
- Klein, G.; MacIntosh, K.; Kaczmarska, I.; Ehrman, J.M. Diatom survivorship in ballast water during trans-Pacific crossings. Biol. Invasions 2010, 12, 1031–1044. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Dickman, M. Mid-ocean exchange of container vessel ballast water. 1: Seasonal factors affecting the transport of harmful diatoms and dinoflagellates. Mar. Ecol. Prog. 1999, 176, 243–251. [Google Scholar] [CrossRef]
- Klouch, K.Z.; Schmidt, S.; Andrieux-Loyer, F.; Le, G.M.; Hervio-Heath, D.; Qui-Minet, Z.N.; Quéré, J.; Bigeard, E.; Guillou, L.; Siano, R. Historical records from dated sediment cores reveal the multidecadal dynamic of the toxic dinoflagellate Alexandrium minutum in the Bay of Brest (France). FEMS Microbiol Ecol. 2016, 92, fiw101. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.; Berge, T.; Lundholm, N.; Andersen, T.J.; Abrantes, F.; Ellegaard, M. Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness. Nat. Commun. 2011, 2, 311. [Google Scholar] [CrossRef] [PubMed]
- Casas-Monroy, O.; Roy, S.; Rochon, A. Ballast sediment-mediated transport of non-indigenous species of dinoflagellates on the East Coast of Canada. Aquat. Invasions 2011, 6, 231–248. [Google Scholar] [CrossRef]
- Klein, G.; Kaczmarska, I.; Ehrman, J.M. The diatom Chaetoceros in ships’ ballast waters - survivorship of stowaways. Acta. Bot. Croat. 2009, 68, 325–338. [Google Scholar]
- Casas-Monroy, O.; Parenteau, M.; Drake, D.A.R.; Roy, S.; Rochon, A. Absolute estimates of the propagule pressure of viable dinoflagellates across Canadian coasts: The variable influence of ballast water exchange. Mar. Biol. 2016, 163, 174. [Google Scholar] [CrossRef]
- Siano, R.; Kooistra, W.; Montresor, M.; Zingone, A. Unarmoured and thin-walled dinoflagellates from the Gulf of Naples, with the description of Woloszynskia cincta sp nov (Dinophyceae, Suessiales). Phycologia 2009, 48, 44–65. [Google Scholar] [CrossRef]
- Bolch, C.J.S.; Negri, A.P.; Hallegraeff, G.M. Gymnodinium microreticulatum sp. nov. (Dinophyceae): A naked, microreticulate cyst-producing dinoflagellate, distinct from Gymnodinium catenatum and Gymnodinium nolleri. Phycologia 1999, 38, 301–313. [Google Scholar] [CrossRef]
- Doblin, M.A.; Popels, L.C.; Coyne, K.J.; Hutchins, D.A.; Cary, S.C.; Dobbs, F.C. Transport of the harmful bloom alga Aureococcus anophagefferens by oceangoing ships and coastal boats. Appl. Environ. Microbiol. 2004, 70, 6495–6500. [Google Scholar] [CrossRef] [PubMed]
- López-García, P.; Rodríguez-Valera, F.; Pedrós-Alió, C.; Moreira, D. Unexpected diversity of small eukaryotes in deep-sea Antarctic plankton. Nature 2001, 409, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Basurko, O.C.; Rodriguez-Ezpeleta, N. The challenges and promises of genetic approaches for ballast water management. J. Sea Res. 2018, 133, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Darling, J.A.; Blum, M.J. DNA-based methods for monitoring invasive species: A review and prospectus. Biol. Invasions 2007, 9, 751–765. [Google Scholar] [CrossRef]
- Garrett, M.J.; Puchulutegui, C.; Selwood, A.I.; Wolny, J.L. Identification of the harmful dinoflagellate Vulcanodinium rugosum recovered from a ballast tank of a globally traveled ship in Port Tampa Bay, Florida, USA. Harmful Algae 2014, 39, 202–209. [Google Scholar] [CrossRef]
- Zaiko, A.; Martinez, J.L.; Schmidt-Petersen, J.; Ribicic, D.; Samuiloviene, A.; Garcia-Vazquez, E. Metabarcoding approach for the ballast water surveillance - An advantageous solution or an awkward challenge? Mar. Pollut. Bull. 2015, 92, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.L.A.; Weyrich, L.S.; Hallegraeff, G.; Cooper, A. Retrospective eDNA assessment of potentially harmful algae in historical ship ballast tank and marine port sediments. Mol. Ecol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Johengen, T.H.; Reid, D.; Fahnenstiel, G.; MacIsaac, H.; Dobbs, F.; Doblin, M.; Ruiz, G.; Jenkins, P. Assessment of Transoceanic NOBOB Vessels and Low-Salinity Ballast Water as Vectors for Nonindigenous Species Introductions to the Great Lakes; University of Michigan and NOAA-Great Lakes Environmental Research Laboratory: Ann Arbor, MI, USA, 2005. [Google Scholar]
- Daugbjerg, N.; Hansen, G.; Larsen, J.; Moestrup, Ø. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of 3 new genera of unarmoured dinoflagellates. Phycologia 2000, 39, 302–317. [Google Scholar] [CrossRef]
- Wylezich, C.; Nies, G.; Mylnikov, A.P.; Tautz, D.; Arndt, H. An evaluation of the use of the LSU rRNA D1-D5 Domain for DNA-based taxonomy of eukaryotic protists. Protist 2010, 161, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolch, C.J.S. The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments. Phycologia 1997, 36, 472–478. [Google Scholar] [CrossRef]
- Litaker, W.R.; Vandersea, M.W.; Kibler, S.R.; Reece, K.S.; Stokes, N.A.; Steidinger, K.A.; Millie, D.F.; Bendis, B.J.; Pigg, R.J.; Tester, P.A. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J. Phycol. 2003, 39, 754–761. [Google Scholar] [CrossRef]
- Bandschmidt, C.J.; Lechugadevéze, C.H.; Kulis, D.M.; Anderson, D.M. Culture studies of Alexandrium affine (Dinophyceae), a non-toxic cyst forming dinoflagellate from Bahía Concepción, gulf of California. Bot. Mar. 2003, 46, 44–54. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, L. An autecological study of the potentially toxic dinoflagellate Alexandrium affine isolated from Vietnamese waters. Harmful Algae 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Fahnenstiel, G.; Hong, Y.; Millie, D.; Doblin, M.; Johengen, T.; Reid, D. Marine dinoflagellate cysts in the ballast tank sediments of ships entering the Laurentian Great Lakes. Int. Assoc. Theor. Appl. Limnol. 2009, 30, 1035–1038. [Google Scholar] [CrossRef] [Green Version]
- John, U.; Litaker, R.W.; Montresor, M.; Murray, S.; Brosnahan, M.L.; Anderson, D.M. Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: The introduction of five species with emphasis on molecular-based (rDNA) classification. Protist 2014, 165, 779–804. [Google Scholar] [CrossRef]
- Kremp, A.; Lindholm, T.; Dreßler, N.; Erler, K.; Gerdts, G.; Eirtovaara, S.; Leskinen, E. Bloom forming Alexandrium ostenfeldii (Dinophyceae) in shallow waters of the Åland Archipelago, Northern Baltic Sea. Harmful Algae 2009, 8, 318–328. [Google Scholar] [CrossRef]
- Boltovskoy, D.; Almada, P.; Correa, N. Biological invasions: Assessment of threat from ballast-water discharge in Patagonian (Argentina) ports. Environ. Sci. Policy 2011, 14, 578–583. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Bravo, I.; Garcés, E. The significance of sexual versus asexual cyst formation in the life cycle of the noxious dinoflagellate Alexandrium peruvianum. Harmful Algae 2008, 7, 653–663. [Google Scholar] [CrossRef]
- Lim, P.T.; Usup, G.; Leaw, C.P.; Ogata, T. First report of Alexandrium taylori and Alexandrium peruvianum (Dinophyceae) in Malaysia waters. Harmful Algae 2005, 4, 391–400. [Google Scholar] [CrossRef]
- Franco, J.M.; Paz, B.; Riobo, P.; Pizarro, G.; Figueroa, R.; Fraga, S.; Bravo, I. First report of the production of spirolides by Alexandrium peruvianum (Dinophyceae) from the mediterranean sea. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006. [Google Scholar]
- Triki, H.Z.; Daly-Yahia, O.K.; Malouche, D.; Komiha, Y.; Deidun, A.; Brahim, M.; Laabir, M. Distribution of resting cysts of the potentially toxic dinoflagellate Alexandrium pseudogonyaulax in recently-deposited sediment within Bizerte Lagoon (Mediterranean coast, Tunisia). Mar. Pollut. Bull. 2014, 84, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Espiña, B.; Cagide, E.; Louzao, M.C.; Vilariño, N.; Vieytes, M.R.; Takeda, Y.; Sasaki, M.; Botana, L.M. Cytotoxicity of goniodomin A and B in non contractile cells. Toxicol. Lett. 2016, 250–251, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Parenteau, M.; Casas-Monroy, O.; Rochon, A. Coastal ship traffic: A significant introduction vector for potentially harmful dinoflagellates in eastern Canada. Can. J. Fish. Aquat. Sci. 2012, 69, 627–644. [Google Scholar] [CrossRef]
- Rengefors, K.; Legrand, C. Toxicity in Peridinium aciculiferum−an adaptive strategy to outcompete other winter phytoplankton? Limnol. Oceanogr. 2001, 46, 1990–1997. [Google Scholar] [CrossRef]
- Craveiro, S.; Daugbjerg, N.; Moestrup, Ø.; Calado, J.A. Studies on Peridinium aciculiferum and P. malmogiense (=Scrippsiella hangoei): Comparison with Chimonodinium lomnickii and description of Apocalathium gen. nov. (Dinophyceae). Phycologia 2016, 56, 21–35. [Google Scholar] [CrossRef]
- Rengefors, K.; Karlsson, I.; Hansson, L. Algal cyst dormancy: A temporal escape from herbivory. Proc. Biol. Sci. 1998, 265, 1353–1358. [Google Scholar] [CrossRef]
- Annenkova, N.V.; Hansen, G.; Moestrup, O.; Rengefors, K. Recent radiation in a marine and freshwater dinoflagellate species flock. ISME J. 2015, 9, 1821–1834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengefors, K.; Meyer, B. Peridinium euryceps sp. nov. (Peridiniales, Dinophyceae), a cryophilic dinoflagellate from Lake Erken, Sweden. Phycologia 1998, 37, 284–291. [Google Scholar] [CrossRef]
- Kremp, A.; Heiskanen, A.-S. Sexuality and cyst formation of the spring-bloom dinoflagellate Scrippsiella hangoei in the coastal northern Baltic Sea. Mar. Biol. 1999, 134, 771–777. [Google Scholar] [CrossRef]
- Hamer, J.P.; McCollin, T.A.; Lucas, I.A.N. Dinoflagellate cysts in ballast tank sediments: Between tank variability. Mar. Pollut. Bull. 2000, 40, 731–733. [Google Scholar] [CrossRef]
- Mertens, K.N.; Yamaguchi, A.; Kawami, H.; Ribeiro, S.; Leander, B.S.; Price, A.M.; Pospelova, V.; Ellegaard, M.; Matsuoka, K. Archaeperidinium saanichi sp. nov.: A new species based on morphological variation of cyst and theca within the Archaeperidinium minutum Jörgensen 1912 species complex. Mar. Micropaleontol. 2012, 96–97, 48–62. [Google Scholar] [CrossRef]
- Tillmann, U.; Soehner, S.; Nézan, E.; Krock, B. First record of the genus Azadinium (Dinophyceae) from the Shetland Islands, including the description of Azadinium polongum sp. nov. Harmful Algae 2012, 20, 142–155. [Google Scholar] [CrossRef]
- Tillmann, U.; Elbrächter, M.; John, U.; Krock, B. A new non-toxic species in the dinoflagellate genus Azadinium: A. poporum sp. nov. Eur. J. Phycol. 2011, 46, 74–87. [Google Scholar] [CrossRef]
- Gu, H.; Luo, Z.; Krock, B.; Witt, M.; Tillmann, U. Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae 2013, 21–22, 64–75. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N.; Henriksen, P. Baldinia anauniensis gen. et sp nov.: A ‘new’ dinotlagellate from Lake Tovel, N. Italy. Phycologia 2007, 46, 86–108. [Google Scholar] [CrossRef]
- Sampedro, N.; Fraga, S.; Penna, A.; Casabianca, S.; Zapata, M.; Grünewald, C.F.; Riobó, P.; Camp, J. Barrufeta bravensis gen. nov. sp. nov. (Dinophyceae): A new bloom-forming species from the northwest Mediterranean Sea. J. Phycol. 2011, 47, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Kremp, A.; Elbrächter, M.; Schweikert, M.; Wolny, J.L.; Gottschling, M. Woloszynskia halophila (Biecheler) comb. nov.: A bloom-forming cold-water dinoflagellate co-occurring with Scrippsiella hangoei (Dinophyceae) in the Baltic Sea. J. Phycol. 2005, 41, 629–642. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Lindberg, K.; Daugbjerg, N. Studies on woloszynskioid dinoflagellates IV: The genus Biecheleria gen. nov. Phycol. Res. 2009, 57, 203–220. [Google Scholar] [CrossRef]
- Takahashi, K.; Sarai, C.; Iwataki, M. Morphology of two marine woloszynskioid dinoflagellates, Biecheleria brevisulcata sp. nov. and Biecheleriopsis adriatica (Suessiaceae, Dinophyceae), from Japanese coasts. Phycologia 2014, 53, 52–65. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Lindberg, K.; Daugbjerg, N. Studies on woloszynskioid dinoflagellates V. Ultrastructure of Biecheleriopsis gen. nov., with description of Biecheleriopsis adriatica sp. nov. Phycol. Res. 2009, 57, 221–237. [Google Scholar] [CrossRef]
- Skovgaard, A.; Massana, R.; Saiz, E. Parasitic species of the Genus Blastodinium (Blastodiniphyceae) are Peridinioid Dinoflagellates. J. Phycol. 2007, 43, 553–560. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Hansen, G.; Daugbjerg, N. Studies on woloszynskioid dinoflagellates III: On the ultrastructure and phylogeny of Borghiella dodgei Gen. et sp. nov., a cold-water species from Lake Tovel, N. Italy, and on B. Tenuissima Comb. Nov. (syn. Woloszynskia Tenuissima). Phycologia 2008, 47, 54–78. [Google Scholar] [CrossRef]
- Craveiro, S.C.; Calado, A.J.; Daugbjerg, N.; Hansen, G.; Moestrup, Ø. Ultrastructure and LSU rDNA-based phylogeny of Peridinium lomnickii and description of Chimonodinium gen. nov. (Dinophyceae). Protist 2011, 162, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Steidinger, K.A.; Landsberg, J.H.; Mason, P.L.; Vogelbein, W.K.; Tester, P.A.; Wayne Litaker, R. Cryptoperidiniopsis brodyi gen. et sp. nov. (Dinophyceae), a small lightly armored dinoflagellate in the Pfiesteriaceae. J. Phycol. 2006, 42, 951–961. [Google Scholar] [CrossRef]
- Gómez, F. A list of free-living dinoflagellate species in the world’s oceans. Acta Bot. Croat. 2005, 64, 129–212. [Google Scholar]
- Drebes, G. Dissodinium pseudolunula (Dinophyta), a parasite on copepod eggs. Br. Phycol. J. 1978, 13, 319–327. [Google Scholar] [CrossRef]
- Harvey, M.; Gilbert, M.; Gauthierl, D.; Reid, D.M. A preliminary assessment of risks for the ballast water-mediated introduction of nonindigenous marine organisms in the Estuary and Gulf of St. Lawrence. J. Geo-Inf. Sci. 1999, 12, 89–94. [Google Scholar]
- Rao, D.V.S.; Sprules, W.G.; Locke, A.; Carlton, J.T. Exotic phytoplankton from ships’ ballast waters: Risk of potential spread to mariculture sites on Canada’s East Coast. Can. Data Fish. Aquatic Sci. 1994, 937, 1–51. [Google Scholar]
- Coats, D.W.; Kim, S.; Bachvaroff, T.R.; Handy, S.M.; Delwiche, C.F. Tintinnophagus acutus n. g., n. sp. (Phylum Dinoflagellata), an ectoparasite of the ciliate Tintinnopsis cylindrica Daday 1887, and its relationship to Duboscquodinium collini Grasse 1952. J. Eukaryot. Microbiol. 2010, 57, 468–482. [Google Scholar] [CrossRef]
- Coats, D.W. Duboscquella cachoni n. sp., a parasitic dinoflagellate lethal to its tintinnine host eutintinnus pectinis. J. Protozool. 1988, 35, 607–617. [Google Scholar] [CrossRef]
- Coats, D.W.; Bachvaroff, T.R.; Delwiche, C.F. Revision of the family Duboscquellidae with description of Euduboscquella crenulata n. gen., n. sp. (Dinoflagellata, Syndinea), an intracellular parasite of the ciliate Favella panamensis Kofoid & Campbell. J. Eukaryot. Microbiol. 2012, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J. Feeding by the mixotrophic red-tide dinoflagellate Gonyaulax polygramma: Mechanisms, prey species, effects of prey concentration, and grazing impact. Aquat. Microb. Ecol. 2005, 38, 249–257. [Google Scholar] [CrossRef]
- Garrett, M.J.; Wolny, J.L.; Williams, B.J.; Dirks, M.D.; Brame, J.A.; Richardson, R.W. Methods for sampling and analysis of marine microalgae in ship ballast tanks: A case study from Tampa Bay, Florida, USA. Algae 2011, 26, 181–192. [Google Scholar] [CrossRef]
- Rhodes, L.; McNabb, P.; de Salas, M.; Briggs, L.; Beuzenberg, V.; Gladstone, M. Yessotoxin production by Gonyaulax spinifera. Harmful Algae 2006, 5, 148–155. [Google Scholar] [CrossRef]
- Hansen, G.; Moestrup, Ø.; Roberts, K.R. Fine structural observations on Gonyaulax spinifera (Dinophyceae), with special emphasis on the flagellar apparatus. Phycologia 1996, 35, 354–366. [Google Scholar] [CrossRef]
- Briski, E.; Gollasch, S.; David, M.; Linley, R.D.; Casas-Monroy, O.; Rajakaruna, H.; Bailey, S.A. Combining ballast water exchange and treatment to maximize prevention of species introductions to freshwater ecosystems. Environ. Sci. Technol. 2015, 49, 9566–9573. [Google Scholar] [CrossRef]
- Oshima, Y.; Blackburn, S.I.; Hallegraeff, G.M. Comparative study on paralytic shellfish toxin profiles of the dinoflagellate Gymnodinium catenatum from three different countries. Mar. Biol. 1993, 116, 471–476. [Google Scholar] [CrossRef]
- Mcminn, A.; Hallegraeff, G.; Thomson, P.; Jenkinson, A.; Heijnis, H. Cyst and radionucleotide evidence for the recent introduction of the toxic dinoflagellate Gymnodinium catenatum into Tasmanian waters. Mar. Ecol. Prog. 1997, 161, 165–172. [Google Scholar] [CrossRef]
- Fraga, S.; Bravo, I.; Delgado, M.; Franco, J.M.; Zapata, M. Gyrodinium impudicum sp nov (Dinophyceae), a non toxic, chain-forming, red tide dinoflagellate. Phycologia 1995, 34, 514–521. [Google Scholar] [CrossRef]
- Oh, S.J.; Kwon, H.K.; Noh, I.H.; Yang, H.-S. Dissolved organic phosphorus utilization and alkaline phosphatase activity of the dinoflagellate Gymnodinium impudicum isolated from the South Sea of Korea. Ocean Sci. J. 2010, 45, 171–178. [Google Scholar] [CrossRef]
- Gu, H.; Liu, T.; Vale, P.; Luo, Z. Morphology, phylogeny and toxin profiles of Gymnodinium inusitatum sp. nov., Gymnodinium catenatum and Gymnodinium microreticulatum (Dinophyceae) from the Yellow Sea, China. Harmful Algae 2013, 28, 97–107. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, W.; Xu, B.; Zheng, B.; Gu, H. Morphology, ultrastructure, and phylogeny of Protodinium simplex and Biecheleriopsis cf. adriatica (Dinophyceae) from the China Sea. Nova Hedwig. 2015, 101, 251–268. [Google Scholar] [CrossRef]
- Zvyagintsev, A.Y.; Ivin, V.V.; Kashin, I.A.; Orlova, T.Y.; Selina, M.S.; Kasyan, V.V.; Korn, O.M.; Kornienko, E.S.; Kulikova, V.A.; Bezverbnaya, I.P.; et al. Acclimation and introduction of hydrobionts ships’ ballast water organisms in the Port of Vladivostok. Russ. J. Mar. Biol. 2009, 35, 41–52. [Google Scholar] [CrossRef]
- Zvyagintsev, A.Y.; Selifonova, J.P. Hydrobiological studies of the ballast waters of cargo ships in Russian Sea ports. Oceanology 2010, 50, 924–932. [Google Scholar] [CrossRef]
- Kang, N.S.; Jeong, H.J.; Moestrup, O.; Park, T.G. Gyrodiniellum shiwhaense n. gen., n. sp., a new planktonic heterotrophic dinoflagellate from the coastal waters of western Korea: Morphology and ribosomal DNA gene sequence. J. Eukaryot. Microbiol. 2011, 58, 284–309. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, S.; Hiromi, J. Growth and grazing of a naked heterotrophic dinoflagellate, Gyrodinium dominans. Aquat. Microb. Ecol. 1995, 9, 157–164. [Google Scholar] [CrossRef]
- Steichen, J.L.; Denby, A.; Windham, R.; Brinkmeyer, R.; Quigg, A. A tale of two ports: Dinoflagellate and diatom communities found in the high ship traffic region of Galveston Bay, Texas (USA). J. Coast. Res. 2015, 31, 407–416. [Google Scholar] [CrossRef]
- Larsen, J. Unarmoured dinoflagellates from Australian waters II. Genus Gyrodinium (Gymnodiniales, Dinophyceae). Phycologia 1996, 35, 342–349. [Google Scholar] [CrossRef]
- Takano, Y.; Horiguchi, T. Surface ultrastructure and molecular phylogenetics of four unarmored heterotrophic dinoflagellates, including the type species of the genus Gyrodinium (Dinophyceae). Phycol. Res. 2004, 52, 107–116. [Google Scholar] [CrossRef]
- Hansen, G.; Daugbjerg, N. Ultrastructure of Gyrodinium spirale, the type species of Gyrodinium (Dinophyceae), including a phylogeny of G. dominans, G. rubrum and G. spirale deduced from partial LSU rDNA sequences. Protist 2004, 155, 271–294. [Google Scholar] [CrossRef]
- Morquecho, L.; Lechugadevéze, C.H. Seasonal occurrence of planktonic dinoflagellates and cyst production in relationship to environmental variables in subtropical Bahı’a Concepción, Gulf of California. Bot. Mar. 2004, 47, 313–322. [Google Scholar] [CrossRef]
- Drebes, G.; Schnepf, E. Gyrodinium undulans Hulburt, a marine dinoflagellate feeding on the bloom-forming diatom Odontella aurita, and on copepod and rotifer eggs. Helgoländer Meeresunters 1998, 52, 1–14. [Google Scholar] [CrossRef]
- Pomroy, A.J. Scanning electron microscopy of Heterocapsa minima sp. nov. (Dinophyceae) and its seasonal distribution in the Celtic Sea. Br. Phycol. J. 1989, 24, 131–135. [Google Scholar] [CrossRef]
- Hansen, G. Analysis of the thecal plate pattern in the dinoflagellate Heterocapsa rotundata (Lohmann) comb. nov. (= Katodinium rotundatum (Lohmann) Loeblich). Phycologia 1995, 34, 166–170. [Google Scholar] [CrossRef]
- Millette, N.C.; King, G.E.; Pierson, J.J. A note on the survival and feeding of copepod nauplii (Eurytemora carolleeae) on the dinoflagellate Heterocapsa rotundata. J. Plankton Res. 2015, 37, 1095–1099. [Google Scholar] [CrossRef]
- Pertola, S.; Faust, M.A.; Kuosa, H. Survey on germination and species composition of dinoflagellates from ballast tanks and recent sediments in ports on the South Coast of Finland, North-Eastern Baltic Sea. Mar. Pollut. Bull. 2006, 52, 900–911. [Google Scholar] [CrossRef]
- Olenin, S.; Gollasch, S.; Jonusas, S.; Rimkute, I. En-route investigations of plankton in ballast water on a ship’s voyage from the Baltic Sea to the open Atlantic coast of Europe. Int. Rev. Hydrobiol. 2000, 85, 577–596. [Google Scholar] [CrossRef]
- Butron, A.; Orive, E.; Madariaga, I. Potential risk of harmful algae transport by ballast waters: The case of Bilbao Harbour. Mar. Pollut. Bull. 2011, 62, 747–757. [Google Scholar] [CrossRef]
- Kawami, H.; Van Wezel, R.; Koeman, R.P.T.; Matsuoka, K. Protoperidinium tricingulatum sp. nov. (Dinophyceae), a new motile form of a round, brown, and spiny dinoflagellate cyst. Phycol. Res. 2009, 57, 259–267. [Google Scholar] [CrossRef]
- Potvin, E.; Rochon, A.; Lovejoy, C. Cyst-theca relationship of the arctic dinoflagellate cyst Islandinium minutum (Dinophyceae) and phylogenetic position based on SSU rDNA and LSU rDNA. J. Phycol. 2013, 49, 848–866. [Google Scholar] [CrossRef]
- Botes, L.; Sym, S.D.; Pitcher, G.C. Karenia cristata sp. nov. and Karenia bicuneiformis sp. nov. (Gymnodiniales, Dinophyceae): Two new Karenia species from the South African Coast. Phycologia 2003, 42, 563–571. [Google Scholar] [CrossRef]
- Haywood, A.J.; Steidinger, K.A.; Truby, E.W.; Bergquist, P.R.; Bergquist, P.L.; Adamson, J.; Mackenzie, L. Coparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand. J. Phycol. 2004, 40, 165–179. [Google Scholar] [CrossRef]
- de Salas, M.F.; Laza-Martinez, A.; Hallegraeff, G.M. Novel unarmored dinoflagellates from the toxigenic family Kareniaceae (Gymnodiniales): Five new species of Karlodinium and one new Takayama from the Australian sector of the Southern Ocean. J. Phycol. 2008, 44, 241–257. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Hakanen, P.; Hansen, G.; Daugbjerg, N.; Ellegaard, M. On Levanderina fissa gen. & comb. nov. (Dinophyceae) (syn. Gymnodinium fissum, Gyrodinium instriatum, Gyr. uncatenum), a dinoflagellate with a very unusual sulcus. Phycologia 2014, 53, 265–292. [Google Scholar] [CrossRef]
- Iwataki, M.; Kawami, H.; Matsuoka, K. Cochlodinium fulvescens sp. nov. (Gymnodiniales, Dinophyceae), a new chain-forming unarmored dinoflagellate from Asian coasts. Phycol. Res. 2007, 55, 231–239. [Google Scholar] [CrossRef]
- Iwataki, M.; Takayama, H.; Takahashi, K.; Matsuoka, K. Taxonomy and distribution of the unarmored dinoflagellates Cochlodinium polykrikoides and C. fulvescens. Mar. Protists 2015, 511–565. [Google Scholar] [CrossRef]
- Chang, S.K.; Lee, S.G.; Chang, K.L.; Kim, H.G.; Jin, J. Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J. Plankton Res. 1999, 21, 2105–2115. [Google Scholar] [CrossRef]
- Holt, J.R. Studies in the dinoflagellate genera, Peridinium and Peridiniopsis. Ph.D. Thesis, The University of Oklahoma, Ann Arbor, MI, USA, 1981. [Google Scholar]
- Craveiro, S.C.; Daugbjerg, N.; Moestrup, Ø.; Calado, A.J. Fine-structural characterization and phylogeny of Peridinium polonicum, type species of the recently described genus Naiadinium (Dinophyceae). Eur. J. Protistol. 2015, 51, 259–279. [Google Scholar] [CrossRef]
- Chomérat, N.; Couté, A.; Fayolle, S.; Mascarell, G.; Cazaubon, A. Morphology and ecology of Oblea rotunda (Diplopsalidaceae, Dinophyceae) from a new habitat: A brackish and hypertrophic ecosystem, the Étang de Bolmon (South of France). Eur. J. Phycol. 2004, 39, 317–326. [Google Scholar] [CrossRef]
- Craveiro, S.C.; Calado, A.J.; Daugbjerg, N.; Moestrup, Ø. Ultrastructure and LSU rDNA-based revision of Peridinium group Palatinum (Dinophyceae) with the description of Palatinus gen. nov. J. Phycol. 2009, 45, 1175–1194. [Google Scholar] [CrossRef]
- Siano, R.; Montresor, M.; Probert, I.; Not, F.; de Vargas, C. Pelagodinium gen. nov. and P. béii comb. nov., a dinoflagellate symbiont of planktonic foraminifera. Protist 2010, 161, 385–399. [Google Scholar] [CrossRef]
- Potvin, É.; Jeong, H.J.; Kang, N.S.; Noh, J.H.; Yang, E.J. Morphology, molecular phylogeny, and pigment characterization of an isolate of the dinoflagellate Pelagodinium bei from Korean waters. Algae 2015, 30, 183–195. [Google Scholar] [CrossRef]
- Onuma, R.; Watanabe, K.; Horiguchi, T. Pellucidodinium psammophilum gen. & sp. nov. and Nusuttodinium desymbiontum sp. nov. (Dinophyceae), two novel heterotrophs closely related to kleptochloroplastidic dinoflagellates. Phycologia 2015, 54, 192–209. [Google Scholar] [CrossRef]
- Gu, H.; Luo, Z.; Zeng, N.; Lan, B.; Lan, D. First record of Pentapharsodinium (Peridiniales, Dinophyceae) in the China Sea, with description of Pentapharsodinium dalei var aciculiferum. Phycol. Res. 2013, 61, 256–267. [Google Scholar] [CrossRef]
- Smith, K.; Dodson, M.; Santos, S.; Gast, R.; Rogerson, A.; Sullivan, B.; Moss, A.G. Pentapharsodinium tyrrhenicum is a parasitic dinoflagellate of the ctenophore Mnemiopsis Leidyi. J. Phycol. 2007, 43, 37. [Google Scholar]
- Montresor, M.; Zingone, A.; Marino, D. The calcareous resting cyst of Pentapharsodinium tyrrhenicum comb. nov. (Dinophyceae). J. Phycol. 1993, 29, 223–230. [Google Scholar] [CrossRef]
- Calado, A.J.; Moestrup, Ø. Ultrastructural study of the type species of Peridiniopsis, Peridiniopsis borgei (Dinophyceae), with special reference to the peduncle and flagellar apparatus. Phycologia 2002, 41, 567–584. [Google Scholar] [CrossRef]
- Montresor, M.; Lovejoy, C.; Orsini, L.; Procaccini, G.; Roy, S. Bipolar distribution of the cyst-forming dinoflagellate Polarella glacialis. Polar Biol. 2003, 26, 186–194. [Google Scholar] [CrossRef]
- Qiu, D.; Huang, L.; Liu, S.; Zhang, H.; Lin, S. Apical groove type and molecular phylogeny suggests reclassification of Cochlodinium geminatum as Polykrikos geminatum. PLoS ONE 2013, 8, e71346. [Google Scholar] [CrossRef]
- Matsuoka, K.; Kawami, H.; Nagai, S.; Iwataki, M.; Takayama, H. Re-examination of cyst–motile relationships of Polykrikos kofoidii Chatton and Polykrikos schwartzii Bütschli (Gymnodiniales, Dinophyceae). Rev. Palaeobot. Palynol. 2009, 154, 79–90. [Google Scholar] [CrossRef]
- Cohen-Fernandez, E.J.; Meave Del Castillo, E.; Salgado Ugarte, I.H.; Pedroche, F.F. Contribution of external morphology in solving a species complex: The case of Prorocentrum micans, Prorocentrum gracile and Prorocentrum sigmoides (Dinoflagellata) from the Mexican Pacific Coast. Phycol. Res. 2006, 54, 330–340. [Google Scholar] [CrossRef]
- Dhib, A.; Fertouna-Bellakhal, M.; Turki, S.; Aleya, L. Driving factors of dinoflagellate cyst distribution in surface sediments of a Mediterranean lagoon with limited access to the sea. Mar. Pollut. Bull. 2016, 112, 303–312. [Google Scholar] [CrossRef]
- David, M.; Gollasch, S.; Cabrini, M.; Perkovic, M.; Bosnjak, D.; Virgilio, D. Results from the first ballast water sampling study in the Mediterranean Sea-the Port of Koper study. Mar. Pollut. Bull. 2007, 54, 53–65. [Google Scholar] [CrossRef]
- Hyun, B.; Shin, K.; Jang, M.-C.; Jang, P.-G.; Lee, W.-J.; Park, C.; Choi, K.-H. Potential invasions of phytoplankton in ship ballast water at South Korean ports. Mar. Freshw. Res. 2016, 67, 1906–1917. [Google Scholar] [CrossRef]
- Masson, D.; Thomas, G.; Genauzeau, S.; Le Moine, O.; Derrien, A. Merchant ships discharging unwanted marine species in close proximity of a French aquaculture area: Risks involved. Mar. Pollut. Bull. 2013, 77, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Mertens, K.N.; Takano, Y.; Gu, H.; Yamaguchi, A.; Pospelova, V.; Ellegaard, M.; Matsuoka, K. Cyst-theca relationship of a new dinoflagellate with a spiny round brown cyst, Protoperidinium lewisiae sp. nov., and its comparison to the cyst of Oblea acanthocysta. Phycol. Res. 2015, 63, 110–124. [Google Scholar] [CrossRef]
- Gribble, K.E. Sexual and asexual processes in Protoperidinium steidingerae Balech (Dinophyceae), with observations on life-history stages of Protoperidinium depressum (Bailey) Balech (Dinophyceae). J. Eukaryot. Microbiol. 2009, 56, 88. [Google Scholar] [CrossRef]
- Kretschmann, J.; Elbrächter, M.; Zinssmeister, C.; Soehner, S.; Kirsch, M.; Kusber, W.-H.; Gottschling, M. Taxonomic clarification of the dinophyte Peridinium acuminatum Ehrenb., ≡ Scrippsiella acuminata, comb. nov. (Thoracosphaeraceae, Peridiniales). Phytotaxa 2015, 220, 239. [Google Scholar] [CrossRef]
- Janofske, D. Scrippsiella trochoidea and Scrippsiella regalis, nov. comb. (peridiniales, dinophyceae): A comparison. J. Phycol. 2000, 36, 178–189. [Google Scholar] [CrossRef]
- Gu, H.; Sun, J.; Kooistra, W.H.C.F.; Zeng, R. Phylogenetic position and morphology of thecae and cysts of Scrippsiella (Dinophyceae) species in the East China Sea. J. Phycol. 2008, 44, 478–494. [Google Scholar] [CrossRef]
- Balech, E. Two new genera of dinoflagellates from California. Biol. Bull. 1959, 116, 195–203. [Google Scholar] [CrossRef]
- de Salas, M.F.; Bolch, C.J.S.; Botes, L.; Nash, G.; Wright, S.W.; Hallegraeff, G.M. Takayama Gen. Nov. (Gymnodiniales, Dinophyceae), a new genus of unarmored dinoflagellates with sigmoid apical grooves, including the description of two new species. J. Phycol. 2003, 39, 1233–1246. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Kong, L.; Morse, R.E.; Gobler, C.J.; Holmes, M.J. Report of a bloom-forming dinoflagellate Takayama acrotrocha (Dinophyceae) from tropical coastal waters of Singapore. J. Phycol. 2012, 48, 455–466. [Google Scholar] [CrossRef]
- Moestrup, Ø.; Hansen, G.; Daugbjerg, N.; Flaim, G.; D’andrea, M. Studies on woloszynskioid dinoflagellates II: On Tovellia sanguinea sp. nov., the dinoflagellate responsible for the reddening of Lake Tovel, N. Italy. Eur. J. Phycol. 2006, 41, 47–65. [Google Scholar] [CrossRef]
- Calado, A.J.; Craveiro, S.C.; Daugbjerg, N.; Moestrup, Ø. Description of Tyrannodinium gen. nov., a freshwater dinoflagellate closely related to the marine Pfiesteria-like species. J. Phycol. 2009, 45, 1195–1205. [Google Scholar] [CrossRef]
- Calado, A.J. On the identity of the freshwater dinoflagellate Glenodinium edax, with a discussion on the genera Tyrannodinium and Katodinium, and the description of Opisthoaulax gen. nov. Phycologia 2011, 50, 641–649. [Google Scholar] [CrossRef]
- Nicholls, K.H. Introduction to the biology and ecology of the freshwater cryophilic dinoflagellate Woloszynskia pascheri causing red ice. Hydrobiologia 2017, 784, 305–319. [Google Scholar] [CrossRef]
- Balech, E.; Tangen, K. Morphology and taxonomy of toxic species in the tamarensis group (Dinophyceae): Alexandrium excavatum (Braarud) comb. nov. and Alexandrium ostenfeldii (Paulsen) comb. nov. Sarsia 1985, 70, 333–343. [Google Scholar] [CrossRef]
- Mooney, B.D.; Salas, M.D.; Hallegraeff, G.M.; Place, A.R. Survey for karlotoxin production in 15 species of gymnodinioid dinoflagellates (Kareniaceae, Dinophyta). J. Phycol. 2009, 45, 164–175. [Google Scholar] [CrossRef]
- Matsuoka, K.; Fukuyo, Y. Technical Guide for Modern Dinoflagellate Cyst Study; WESTPAC-HAB/WEATPAC/IOC; Japan Society for the Promotion of Science: Tokyo, Japan, 2000; pp. 6–9. [Google Scholar]
- Li, Z.; Han, M.-S.; Matsuoka, K.; Kim, S.-Y.; Shin, H.H. Identification of the resting cyst of Cochlodinium polykrikoides Margalef (Dinophyceae, Gymnodiniales) in Korean coastal sediments. J. Phycol. 2015, 51, 204–210. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Gobler, C.J. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae 2012, 20, 71–80. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Martiny, A.C. Techniques for quantifying phytoplankton biodiversity. Annu. Rev. Mar. Sci. 2015, 7, 299–324. [Google Scholar] [CrossRef]
- Brand, L.E. The salinity tolerance of forty-six marine phytoplankton isolates. Estuar. Coast. Shelf Sci. 1984, 18, 543–556. [Google Scholar] [CrossRef]
- Sullivan, B.E.; Andersen, R.A. Salinity tolerances of 62 strains of Pflesteria and Pfiesteria-like heterotrophic flagellates (Dinophyceae). Phycol. Res. 2001, 49, 207–214. [Google Scholar] [CrossRef]
- Adebayo, A.A.; Zhan, A.B.; Bailey, S.A.; MacIsaac, H.J. Domestic ships as a potential pathway of nonindigenous species from the Saint Lawrence River to the Great Lakes. Biol. Invasions 2014, 16, 793–801. [Google Scholar] [CrossRef]
- Baek, S.H.; Jung, S.W.; Jang, M.C.; Hyun, B.; Shin, K. Survival potential of autotrophic phytoplankton species collected from ballast water in international commercial ships. N. Z. J. Mar. Freshw. Res. 2012, 46, 125–136. [Google Scholar] [CrossRef]
- Pistocchi, R.; Guerrini, F.; Pezzolesi, L.; Riccardi, M.; Vanucci, S.; Ciminiello, P.; Dell’Aversano, C.; Forino, M.; Fattorusso, E.; Tartaglione, L.; et al. Toxin Levels and Profiles in Microalgae from the North-Western Adriatic Sea-15 Years of Studies on Cultured Species. Mar. Drugs 2012, 10, 140–162. [Google Scholar] [CrossRef]
- Cusick, K.D.; Sayler, G.S. An Overview on the Marine Neurotoxin, Saxitoxin: Genetics, Molecular Targets, Methods of Detection and Ecological Functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [Green Version]
- Okolodkov, Y.B. The global distributional patterns of toxic, bloom dinoflagellates recorded from the Eurasian Arctic. Harmful Algae 2005, 4, 351–369. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in Understanding Harmful Algal Blooms: Paradigm Shifts and New Technologies for Research, Monitoring, and Management. Ann. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef] [Green Version]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Andersen, A.J.C.; Andersen, N.G.; Nielsen, K.F.; Hansen, P.J.; Larsen, T.O. Chemical Diversity, Origin, and Analysis of Phycotoxins. J. Nat. Prod. 2016, 79, 662–673. [Google Scholar] [CrossRef] [Green Version]
- Twiner, M.J.; Rehmann, N.; Hess, P.; Doucette, G.J. Azaspiracid shellfish poisoning: A review on the chemistry, ecology, and toxicology with an emphasis on human health impacts. Mar. Drugs 2008, 6, 39–72. [Google Scholar] [CrossRef]
- Otero, A.; Chapela, M.-J.; Atanassova, M.; Vieites, J.M.; Cabado, A.G. Cyclic imines: Chemistry and mechanism of action: A review. Chem. Res. Toxicol. 2011, 24, 1817–1829. [Google Scholar] [CrossRef]
- Graham, H.W. Gymnodinium catenatum, a new dinoflagellate from the gulf of California. Trans. Am. Microsc. Soc. 1943, 62, 259–261. [Google Scholar] [CrossRef]
- Mee, L.D.; Espinosa, M.; Diaz, G. Paralytic shellfish poisoning with a Gymnodinium catenatum red tide on the Pacific Coast of Mexico. Mar. Environ. Res. 1986, 19, 77–92. [Google Scholar] [CrossRef]
- Oshima, Y.; Hasegawa, M.; Yasumoto, T.; Hallegraeff, G.; Blackburn, S. Dinoflagellate Gymnodinium catenatum as the source of paralytic shellfish toxins in Tasmanian shellfish. Toxicon 1987, 25, 1105–1111. [Google Scholar] [CrossRef]
- Ribeiro, S.; Amorim, A.; Andersen, T.J.; Abrantes, F.; Ellegaard, M. Reconstructing the history of an invasion: The toxic phytoplankton species Gymnodinium catenatum in the Northeast Atlantic. Biol. Invasions 2012, 14, 969–985. [Google Scholar] [CrossRef]
- Gárate-Lizárraga, I.; Hernández-Orozco, M.L.; Band-Schmidt, C.J.; Serrano-Casillas, G. Red tides along the coasts of Baja California Sur, México (1984 to 2001). Oceanides 2001, 16, 127–134. [Google Scholar]
- Grindley, J.R.; Taylor, F.J.R. Red water and mass-mortality of fish near Cape Town. Nature 1962, 195, 1324. [Google Scholar] [CrossRef]
- Kudela, R.M.; Gobler, C.J. Harmful dinoflagellate blooms caused by Cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 2012, 14, 71–86. [Google Scholar] [CrossRef]
- Shahraki, J.; Motallebi, A.; Pourahmad, J. Oxidative mechanisms of fish hepatocyte toxicity by the harmful dinoflagellate Cochlodinium polykrikoides. Mar. Environ. Res. 2013, 87, 52–60. [Google Scholar] [CrossRef]
- Dorantes-Aranda, J.J.; García-de la Parra, L.M.; Alonso-Rodríguez, R.; Morquecho, L.; Voltolina, D. Toxic effect of the harmful dinoflagellate Cochlodinium polykrikoides on the spotted rose snapper Lutjanus guttatus. Environ. Toxicol. 2010, 25, 319–326. [Google Scholar] [CrossRef]




| Sample ID | Sampling Date | Sampling Port | Ship Code | Tank Type | Temperature °C | Salinity |
|---|---|---|---|---|---|---|
| 9036 | 19-Nov-01 | Burns Harbor, Indiana, US | 1017 | DBT | 11.7 | 35.0 |
| 9037 | 19-Nov-01 | Burns Harbor, Indiana, US | 1017 | DBT | 11.7 | 35.0 |
| 9043 | 29-Nov-01 | Hamilton, Ontario, Canada | 1020 | FPK | 8.3 | 7.0 |
| 9044 | 13-Jun-02 | Hamilton, Ontario, Canada | 1023 | FPK | 18.5 | 8.0 |
| 9045 | 13-Jun-02 | Hamilton, Ontario, Canada | 1023 | DBT | 18.1 | 2.0 |
| 9046 | 13-Jun-02 | Hamilton, Ontario, Canada | 1023 | DBT | 18.2 | 6.0 |
| 9047 | 24-Jun-02 | Windsor, Ontario, Canada | 1024 | DBT | 21.0 | 16.0 |
| 9048 | 24-Jun-02 | Windsor, Ontario, Canada | 1024 | DBT | 21.0 | 26.0 |
| 9050 | 19-Jul-02 | Hamilton, Ontario, Canada | 1025 | DBT | 21.1 | 26.0 |
| 9051 | 25-Jul-02 | Cleveland, Ohio, US | 1026 | FPK | 22.0 | 4.0 |
| 9054 | 6-Aug-02 | Hamilton, Ontario, Canada | 1027 | DBT | 22.2 | 5.0 |
| 9055 | 6-Aug-02 | Hamilton, Ontario, Canada | 1027 | DBT | 21.9 | 8.0 |
| 9056 | 6-Aug-02 | Hamilton, Ontario, Canada | 1027 | FPK | 23.3 | 2.0 |
| 9057 | 6-Aug-02 | Detroit, Michigan, US | 1028 | FPK | N/A | N/A |
| 9058 | 13-Aug-02 | Windsor, Ontario, Canada | 1029 | DBT | 24.5 | 8.0 |
| 9059 | 13-Aug-02 | Windsor, Ontario, Canada | 1029 | DBT | 24.5 | 3.0 |
| 9060 | 15-Aug-02 | Hamilton, Ontario, Canada | 1030 | DBT | N/A | 31.0 |
| 9061 | 5-Oct-02 | Windsor, Ontario, Canada | 1027 | FPK | 20.2 | 36.0 |
| 9064 | 20-Oct-02 | Windsor, Ontario, Canada | 1033 | FPK | 11.5 | 2.0 |
| 9065 | 20-Oct-02 | Windsor, Ontario, Canada | 1033 | ST | 10.9 | 1.0 |
| 9067 | 23-Oct-02 | Burns Harbor, Indiana, US | 1007 | DBT | 13.6 | 1.1 |
| 9068 | 12-Nov-02 | Cleveland, Ohio, US | 1014 | DBT | 12.3 | 26.0 |
| 9069 | 12-Nov-02 | Cleveland, Ohio, US | 1014 | DBT | 12.3 | 2.0 |
| 9070 | 19-Nov-02 | Cleveland, Ohio, US | 1013 | DBT | 9.8 | 21.0 |
| 9071 | 19-Nov-02 | Cleveland, Ohio, US | 1013 | DBT | 9.2 | 20.6 |
| 9072 | 26-Nov-02 | Hamilton, Ontario, Canada | 1034 | DBT | 7.4 | 28.0 |
| 9073 | 6-Dec-02 | Windsor, Ontario, Canada | 1035 | FPK | -0.7 | 34.0 |
| CB9006 | 16-May-03 | Chesapeake, Virginia, US | N/A | FPK | N/A | N/A |
| CB9009 | 30-Jul-03 | Chesapeake, Virginia, US | N/A | FPK | N/A | N/A |
| CB9012 | 3-Oct-03 | Norfolk, Virginia, US | N/A | FPK | N/A | N/A |
| CB9014 | 21-Nov-03 | Chesapeake, Virginia, US | N/A | FPK | N/A | N/A |
| UNK9074 | 13-Aug-02 | Great Lakes | N/A | N/A | N/A | N/A |
| Species | Synonyms | Habitat | Cyst | Harmful Effects | Reported in BS References | Reported in BW References |
|---|---|---|---|---|---|---|
| Alexandrium affine (1995) | Protogonyaulax affinis (1985) | M [46] | Y [46] | B/T [47] | Reported as Alexandrium affinis [36,48] | N |
| Alexandrium fundyense (1985) | Alexandrium tamarense species complex group I, Alexandrium tamarense (1995) | M [49] | Y [49] | B/T [49] | N | N |
| Alexandrium ostenfeldii (1985) | Protogonyaulax ostenfeldii (1985), Triadinium ostenfeldii (1981), Gessnerium ostenfeldii (1979), Heteraulacus ostenfeldii (1970), Gonyaulax ostenfeldii (1949), Goniodoma ostenfeldii (1904) | M [50] | Y [50] | B/T [50] | N | [26] |
| Alexandrium pacificum (2014) | Alexandrium tamarense species complex group IV, Alexandrium catenella | M [49] | Y [49] | B/T [49] | Reported as A. catenella [10,36] | Reported as A. catenella [21,51] |
| Alexandrium peruvianum (1985) | M [52] | Y [52] | B/T [53,54] | N | N | |
| Alexandrium pseudogonyaulax (1992) | Goniodoma pseudogonyaulax (1952), Triadinium pseudogonyaulax (1981) | M [55] | Y [55] | B/T [56] | [15] | [26,57] |
| Apocalathium aciculiferum (2016) | Peridinium aciculiferum (1900) | F/C [58,59] | Y [58,60] | T [58] | N | Reported as Peridinium aciculiferum [16] |
| Apocalathium baicalense (2016) | Peridinium baicalense (1935) | F [59,61] | Y [61] | N | N | |
| Apocalathium euryceps (2016) | Peridinium euryceps (1998) | F [59,62] | Y [62] | N | N | |
| Apocalathium malmogiense (2016) * | Scrippsiella hangoei (1995) | M/C [59,63] | Y [63] | B [63] | Reported as Scrippsiella hangoei [15,64] | N |
| Archaeperidinium saanichi (2012) | M [65] | Y [65] | N | N | ||
| Azadinium polongum (2012) | M [66] | Y [66] | T [66] | N | N | |
| Azadinium poporum (2011) | M [67] | Y [68] | T [67,68] | N | N | |
| Baldinia anauniensis (2007) | F [69] | Y [69] | B [69] | N | N | |
| Barrufeta bravensis (2011) | M [70] | Y [70] | B [70] | N | N | |
| Biecheleria baltica (2009) | Woloszynskia halophila (2005), Gymnodinium halophilum (1952) | F/Br/M/C [71,72] | Y [71] | B [71] | N | N |
| Biecheleria brevisulcata (2014) | M [73] | Y [73] | N | N | ||
| Biecheleria cincta (2012) | Woloszynskia cincta (2009) | M [27] | Y [27] | N | N | |
| Biecheleriopsis adriatica (2009) | M [74] | Y [74] | N | N | ||
| Blastodinium contortum (1908) | M [75] | N | P [75] | N | N | |
| Borghiella dodgei (2008) | F/C [76] | Y [76] | B [76] | N | N | |
| Borghiella tenuissima (2008) | Woloszynskia tenuissima (1950), Gymnodinium tenuissimum (1894) | F/C [76] | Y [76] | B [76] | N | N |
| Chimonodinium lomnickii (2011) | Glenodinium lomnickii (1928), Peridinium lomnickii (1916) | F/C [77] | Y [77] | B [77] | N | N |
| Cryptoperidiniopsis brodyi (2006) | Br [78] | Y [78] | N | N | ||
| Dinophysis lativelata (1967) | M [79] | N | N | N | ||
| Dissodinium pseudolunula (1978) | M [80] | Y [80] | P [80] | [64] | [26,81,82] | |
| Duboscquodinium collinii (1952) | M [83] | N | P [83] | N | N | |
| Euduboscquella cachoni (2012) | Duboscquella cachoni (1988) | M [84] | N | P [84] | N | N |
| Euduboscquella crenulata (2012) | M [85] | N | P [85] | N | N | |
| Gonyaulax polygramma (1883) | M [86] | N | B/T [86] | N | [26,57,87] | |
| Gonyaulax spinifera (1866) | Peridinium spiniferum (1859) | M [88,89] | Y [88,89] | B/T [88,89] | Reported as Gonyaulax spinifera complex [10,15,17,24,36,48,87] and Spiniferities bentori [14] | [16,26,57,87,90] |
| Gymnodinium catenatum (1943) | M [91] | Y [92] | B [92]/T [91] | [14,17,24,36,48] | N | |
| Gymnodinium impudicum (2000) | Gyrodinium impudicum (1995) | M [93] | Y [94] | B [93] | [17] | N |
| Gymnodinium microreticulatum (1999) | M [28,95] | Y [28,95] | N | N | ||
| Gymnodinium simplex (1921) | Protodinium simplex (1908) | M [96] | N | B [96] | N | [97,98] |
| Gyrodiniellum shiwhaense (2011) | M [99] | N | N | N | ||
| Gyrodinium dominans (1957) | M [100] | N | N | [101] | ||
| Gyrodinium heterogrammum (1996) | M [102] | N | N | N | ||
| Gyrodinium rubrum (2004) | M [103] | N | N | N | ||
| Gyrodinium spirale (1921) | Gymnodinium spirale (1881) | F/Br/M [90,104] | Y [105] | [87] | [26,51,57] | |
| Gyrodinium undulans (1957) | M [106] | Y [106] | N | N | ||
| Heterocapsa minima (1989) | M [107] | N | B [107] | N | N | |
| Heterocapsa rotundata (1995) | Katodinium rotundatum (1965), Massartia rotundata (1933), Amphidinium rotundatum (1908) | M [108] | Y [108] | B [108,109] | [110] | [6,16,101] |
| Heterocapsa triquetra (1883) | Glenodinium triquetrum (1840), Peridinium triquetra (1925) | M [108] | Y [108] | B [108] | [15,81] | [16,26,51,57,90,111,112] |
| Islandinium tricingulatum (2013) | Protoperidinium tricingulatum (2009) | M [113,114] | Y [113] | N | N | |
| Karenia cristata (2003) | M [115] | N | B/T [115] | N | N | |
| Karenia papilionacea (2004) | M [116] | N | B [116]/T [2] | N | [112] | |
| Karlodinium antarcticum (2008) | M [117] | N | T [117] | N | N | |
| Levanderina fissa (2014) | Gymnodinium instriatum (2002), Gyrodinium instriatum (1963), Gyrodinium fissum (1921), Gymnodinium fissum (1894) | M/Br [118] | Y [118] | N | N | |
| Margalefidinium fulvescens (2017) | Cochlodinium fulvescens (2007) | M [18,119] | N | B/T [120] | N | N |
| Margalefidinium polykrikoides (2017)* | Cochlodinium polykrikoides (1961) | M [18,120] | Y [103] | B/T [121] | Reported as Cochlodinium polykrikoides [17,24] | N |
| Naiadinium polonicum (2015) | Peridiniopsis polonicum (1968) | F [122,123] | Y [122] | B [122] | N | N |
| Oblea rotunda (1973) | Peridiniopsis rotunda (1922) | M/Br [124] | Y [124] | [15,110] | [26,57,111] Reported as Oblea rotundata [97,98] | |
| Palatinus apiculatus (2009) | Peridinium palatinum (1896), Peridinium apiculatum (1859), Glenodinium apiculatum (1838) | F [125] | Y [125] | N | N | |
| Pelagodinium beii (2010) | Gymnodinium beii (1987) | M [126,127] | N | N | N | |
| Pellucidodinium psammophilum (2015) | M [128] | N | N | N | ||
| Pentapharsodinium dalei (1986) | M [129] | Y [129] | [14,15,17,24,64] | N | ||
| Pentapharsodinium tyrrhenicum (1993) | Peridinium tyrrhenicum (1990) | M [130] | Y [131] | B/P [130] | [15] | N |
| Peridiniopsis borgei (1904) | Glenodinium borgei (1937), Peridinium borgei (1910) | F [132] | Y [132] | N | N | |
| Polarella glacialis (1999) | M [133] | Y [133] | B [133] | N | N | |
| Polykrikos geminatum (2013) | Cochlodinium geminatum (1896), Gymnodinium geminatum (1895) | M [134] | N | B [134] | N | N |
| Polykrikos kofoidii (1914) | M [135] | Y [135] | [10,17] | [26,57,82,87] | ||
| Prorocentrum micans (1834) | M [136] | N [137] | B/T [136] | [81] | [16,21,26,51,57,82,87,90,112,138,139,140] | |
| Protoperidinium monovelum (1974) | Peridinium monovelum (1936) | M [141] | N | N | N | |
| Protoperidinium steidingerae (1979) | M [142] | Y [142] | N | N | ||
| Scrippsiella acuminata (2015) | Glenodinium acuminatum (1899), Goniodoma acuminatum (1883), Heteraulacus acuminatus (1850), Peridinium acuminatum (1836), Scrippsiella trochoidea (1976) | M [143,144] | Y [143,144] | B [144] | Reported as Scrippsiella trochoidea [10,14,15,17,24,36,48,64,81] | Reported as Scrippsiella trochoidea [16,21,26,51,57,82,90,139] |
| Scrippsiella donghaienis (2008) | M [145] | Y [145] | N | N | ||
| Scrippsiella sweeneyae (1965) | M [146] | Y [146] | N | N | ||
| Takayama acrotrocha (2003) | Gyrodinium acrotrochum (1996) | M [147] | N | B [148] | N | N |
| Takayama helix (2003) | M [147] | N | T [147] | N | N | |
| Tovellia sanguinea (2006) | F [149] | Y [149] | B [149] | N | N | |
| Tyrannodinium edax (2011) | Tyrannodinium berolinense (2009) | F [150,151] | Y [150] | B [150] | N | N |
| Woloszynskia pascheri (1973) | Gymnodinium pascheri (1954), Gyrodinium pascheri (1933), Glenodinium pascheri (1916) | F/C [152] | Y [152] | B [152] | N | N |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, L.; Hu, Z.; Deng, Y.; Liu, Y.; Zhai, X.; Chai, Z.; Liu, X.; Zhan, Z.; Dobbs, F.C.; Tang, Y.Z. Metagenomic Sequencing Identifies Highly Diverse Assemblages of Dinoflagellate Cysts in Sediments from Ships’ Ballast Tanks. Microorganisms 2019, 7, 250. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7080250
Shang L, Hu Z, Deng Y, Liu Y, Zhai X, Chai Z, Liu X, Zhan Z, Dobbs FC, Tang YZ. Metagenomic Sequencing Identifies Highly Diverse Assemblages of Dinoflagellate Cysts in Sediments from Ships’ Ballast Tanks. Microorganisms. 2019; 7(8):250. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7080250
Chicago/Turabian StyleShang, Lixia, Zhangxi Hu, Yunyan Deng, Yuyang Liu, Xinyu Zhai, Zhaoyang Chai, Xiaohan Liu, Zifeng Zhan, Fred C. Dobbs, and Ying Zhong Tang. 2019. "Metagenomic Sequencing Identifies Highly Diverse Assemblages of Dinoflagellate Cysts in Sediments from Ships’ Ballast Tanks" Microorganisms 7, no. 8: 250. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms7080250