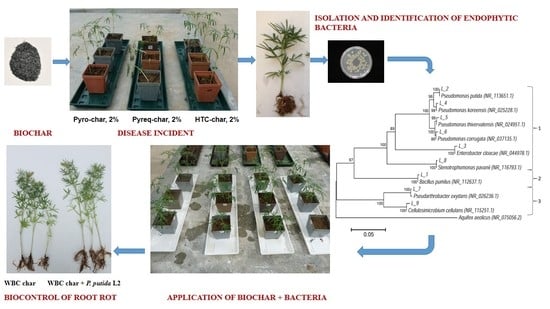
The Effect of Biochars and Endophytic Bacteria on Growth and Root Rot Disease Incidence of Fusarium Infested Narrow-Leafed Lupin (Lupinus angustifolius L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant, Biochar, and Soil
2.2. Plant Growth Experiment
- i)
- plants grown in soil without WBC, MBC, or HTC biochar,
- ii)
- plants grown in soil amended with WBC,
- iii)
- plants grown in soil amended with MBC,
- iv)
- plants grown in soil amended with HTC.
2.3. Isolation of Fungi from Diseased Plants
2.4. Isolation of Endophytic Bacteria from Lupin Roots and Characterization
2.4.1. DNA Isolation
2.4.2. Polymerase Chain Reaction (PCR)
2.4.3. Restriction Fragment Length Polymorphism (RFLP) Analysis
2.4.4. Sequencing and Phylogenetic Analysis
2.5. Plant Growth Traits
2.6. Biological Control of Lupin Root Rot
- -
- un-inoculated plants grown without WBC or MBC biochar,
- -
- un-inoculated plants grown in soil amended with WBC,
- -
- un-inoculated plants grown in soil amended with MBC,
- -
- inoculated plants with Pseudomonas putida L2 grown in soil without WBC or MBC chars,
- -
- inoculated plants with P. putida L2 grown in soil amended with WBC char,
- -
- inoculated plants with P. putida L2 grown in soil amended with MBC char,
- -
- inoculated plants with Stenotrophomonas pavanii L8 grown in soil without WBC or MBC chars,
- -
- inoculated plants with S. pavanii L8 grown in soil amended with WBC char,
- -
- inoculated plants with S. pavanii L8 grown in soil amended with MBC char.
2.7. Plant Growth Experiment with Endophytic Bacteria and Biochar
2.8. Survival of Endophytic Bacterial Strains
2.9. Statistical Analysis
3. Results
3.1. Response of Lupin to the Biochar Types
3.2. Fungal and Bacterial Isolates
3.3. Biological Control of Lupin Root Rot under Biochar Amended Soil
3.4. Plant Growth, Nodulation, and Bacterial Survival in the Root System
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Barrow, C.J. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biederma, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Graber, E.R.; Meller-Harel, Y.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Abd_Allah, E.F.; Berg, G. Biochar treatment resulted in a combined effect on soybean growth promotion and a shift in plant growth promoting rhizobacteria. Front. Microbiol. 2016, 7, 209. [Google Scholar] [CrossRef] [Green Version]
- Islami, T.; Curitno, B.; Basuki, N.; Suryanto, A. Maize yield and associated soil quality changes in cassava + maize intercropping system after 3 years of biochar application. J. Agric. Food. Techn. 2011, 1, 112–115. [Google Scholar]
- Alburquerque, J.A.; Salazar, P.; Barrón, V.; Torrent, J.; del Campillo, M.D.; Gallardo, A.; Villar, R. Enhanced wheat yield by biochar addition under different mineral fertilization levels. Agron. Sustain. Dev. 2013, 33, 475. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Reckling, M.; Wirth, S. Biochar-based inoculum of Bradyrhizobium sp. improves plant growth and yield of lupin (Lupinus albus L.) under drought stress. Eur. J. Soil Biol. 2017, 78, 38–42. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Yu, O.Y.; Raichle, B.; Sink, S. Impact of biochar on the water holding capacity of loamy sand soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Elad, Y.; David, D.R.; Harel, Y.M.; Borenshtein, M.; Silber, K.S.A.; Graber, E.R. Induction of systemic resistance in plants by biochar, a soil applied carbon sequestering agent. Phytopathology 2010, 100, 913–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, A.; Hage-Ahmed, K.; Soja, G.; Steinkellner, S. Potential of Fusarium wilt-inducing chlamydospores, in vitro behaviour in root exudates and physiology of tomato in biochar and compost-amended soil. Plant Soil 2016, 406, 425–440. [Google Scholar] [CrossRef] [Green Version]
- Mehari, Z.H.; Elad, Y.; Rav-David, D.; Graber, E.R.; Harel, Y.M. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 2015, 395, 31–44. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Graber, E.R.; Frenkel, O. Rhizoctonia solani suppression and plant growth promotion in cucumber as affected by biochar pyrolysis temperature, feedstock and concentration. Soil Biol. Biochem. 2014, 69, 110–118. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zheng, J.; Zhang, B.; Lu, H.; Chi, Z.; Pan, G.; Li, L.; Zhen, J.; Zhang, X.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Kolton, M.; Meller Harel, Y.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Berg, G.; Martinez, J.L. Friends or foes: can we make a distinction between beneficial and harmful strains of the Stenotrophomonas maltophilia complex? Front. Microbiol. 2015, 6, 241. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front. Plant Sci. 2016, 7, 1089. [Google Scholar]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Chebotar, V.; Tikhonovich, I.; Kamilova, F.; Validov, S.; et al. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fert. Soils 2011, 47, 197–205. [Google Scholar] [CrossRef]
- Noreen, R.; Ali, S.A.; Hasan, K.A.; Sultana, V.; Ara, J.; Ehteshamul-Haque, S. Evaluation of biocontrol potential of fluorescent Pseudomonas associated with root nodules of mungbean. Crop Prot. 2015, 75, 18e24. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H.Y. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biocharcharacteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237, 105–116. [Google Scholar] [CrossRef]
- Frenkel, O.; Jaiswal, A.K.; Elad, Y.; Lew, B.; Kammann, C.; Graber, E.R. The effect of biochar on plant diseases: what should we learn while designing biochar substrates? J. Environ. Eng. Landsc. 2017, 25, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Gresta, F.; Wink, M.; Prins, U.; Abberton, M.; Capraro, J.; Scarafoni, A.; Hill, G. Lupins in European cropping systems. In Legumes in cropping systems; Murphy-Bokern, D., Stoddard, F., Watson, C., Eds.; CABI Publishing: Wallingford, UK, 2017; pp. 88–108. [Google Scholar]
- Lupwayi, N.Z.; Kennedy, A.C. Grain Legumes in Northern Great Plains. Agron. J. 2007, 99, 1700–1709. [Google Scholar] [CrossRef]
- Reckling, M.; Bergkvist, G.; Watson, C.A.; Stoddard, F.L.; Zander, P.M.; Walker, R.; Pristeri, A.; Toncea, I.; Bachinger, J. Trade-offs between economic and environmental impacts of introducing legumes into cropping systems. Front. Plant Sci. 2016, 7, 669. [Google Scholar]
- Watson, C.; Reckling, M.; Preissel, S.; Bachinger, J.; Bergkvist, G.; Kuhlman, T.; Lindström, K.; Nemecek, T.; Topp, C.; Vanhatalo, A.; et al. Grain legume production and use in European agricultural systems. Adv. Agron. 2017, 144, 235–303. [Google Scholar]
- Jensen, B.; Bødker, L.; Larsen, J.; Knudsen, J.C.H.; Jørnsgaard, B. Specificity of soil-borne pathogens on grain legumes. In Proceedings of the 5th European Conference on Grain Legumes, Dijon, France, 7–11 June 2004. [Google Scholar]
- Reckling, M.; Döring, T.F.; Bergkvist, G.; Stoddard, F.L.; Watson, C.A.; Seddig, S.; Chmielewski, F.-M.; Bachinger, J. Grain legume yields are as stable as other spring crops in long-term experiments across northern Europe. Agron. Sustain. Dev. 2018, 38, 63. [Google Scholar] [CrossRef] [Green Version]
- Reibe, K.; Götz, K.P.; Ross, C.L.; Doering, T.F.; Ellmer, F.; Ruess, L. Impact of quality and quantity of biochar and hydrochar on soil collembola and growth of spring wheat. Soil Biol. Biochem. 2015, 8, 84–87. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Li, L.; Ma, H.; Wirth, S.; Bellingrath-Kimura, S.D. Soil amendments with different maize biochars, to varying degrees, improve chickpea growth under drought by improving symbiotic performance with Mesorhizobium ciceri and soil biochemical properties. Front. Microbiol. 2019, 10, 2423. [Google Scholar] [CrossRef]
- Zeng, Q.Y.; Wang, X.R.; Blomquist, G. Development of mitochondrial ssu rDNA-based oligonucleotide probes for specific detection of common airborne fungi. Mol. Cell. Probes. 2003, 17, 281–288. [Google Scholar] [CrossRef]
- Dashti, A.A.; Jadaon, M.M.; Abdulsamad, A.M.; Dashti, H.M. Heat Treatment of Bacteria: A simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009, 41, 117–122. [Google Scholar]
- Jinneman, K.C.; Wetherington, J.H.; Adams, A.M.; Johnson, J.M.; Tenge, B.J.; Dang, N.L.; Hill, W.E. Differentiation of Cyclospora sp. and Eimeria spp. by Using the Polymerase Chain Reaction Amplification Products and Restriction Fragment Length Polymorphisms; Food and Drug Admin Lab Information Bulletin LIB no. 4044: Cebu, Philippines, 1996. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Nat. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Bano, N.; Musarrat, J. Characterization of a new Pseudomonas aeruginosa strain NJ-15 as a potential biocontrol agent. Curr. Microbiol. 2003, 46, 324–328. [Google Scholar] [CrossRef]
- Malleswari, D.; Bagyanarayan, G. In vitro screening of rhizobacteria isolated from the rhizosphere of medicinal and aromatic plants for multiple plant growth promoting activities. J. Microbiol. Biotechnol. Res. 2013, 3, 84–89. [Google Scholar]
- Gupta, P.; Samant, K. SahuIsolation of cellulose-degrading bacteria and determination of their cellulolytic potential. Int. J. Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glandorf, D.C.M.; Brand, I.; Bakker, P.A.H.M.; Schippers, B. Stability of rifampicin resistance as a marker for root colonization studies of Pseudomonas putida in the field. Plant Soil 1992, 146, 135–142. [Google Scholar] [CrossRef]
- Höflich, G.; Wiehe, W.; Hecht-Buchholz, C.H. Rhizosphere colonization of different growth promoting Pseudomonas and Rhizobium bacteria. Microbiol. Res. 1995, 150, 139–147. [Google Scholar] [CrossRef]
- Harel, Y.M.; Elad, Y.; Rav-David, D.; Borenstein, M.; Shulchani, R.; Lew, B.; Graber, E.R. Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 2012, 357, 245–257. [Google Scholar] [CrossRef]
- Graber, E.R.; Elad, Y. Biochar impact on plant resistance to disease. In Biochar and Soil Biota; Ladygina, N., Rineau, F., Eds.; CRC Press (Taylor and Francis Group): Boca Raton, FL, USA, 2013; pp. 41–68. [Google Scholar]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, H.; Hook, J.; van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Shen, Q.; Hedley, M.; Camps Arbestain, M.; Kirschbaum, M.U.F. Can biochar increase the bioavailability of phosphorus? J. Soil Sci. Plant Nutr. 2016, 16, 268–286. [Google Scholar] [CrossRef] [Green Version]
- Bonanomi, G.; Ippolito, F.; and Scala, F. A “black” future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J. Plant Pathol. 2015, 97, 223–234. [Google Scholar]
- Sanogo, S.; Yang, X.B. Relation of sand content, pH, and potassium and phosphorus nutrition to the development of sudden death syndrome in soybean. Can. J. Plant Pathol. 2001, 23, 174–180. [Google Scholar] [CrossRef]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases–review and future prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Shurigin, V.; Hashem, A.; Abd_Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 28, 1887. [Google Scholar] [CrossRef]
- Silva, M.C.; Nicole, M.; Rijo, L.; Geiger, J.P.; Rodrigues, C.J., Jr. Cytochemical aspects of the plant-rust fungus interface during the compatible interaction Coffea arabica (cv. Caturra)-Hemileia vastatrix (race III). Int. J. Plant Sci. 1999, 160, 79–91. [Google Scholar]
- Abdallah, R.A.B.; Jabnoun-Khiareddine, J.; Nefzi, A.; Mokni-Tlili, S.; Daami-Remadi, M. Biocontrol of Fusarium wilt and growth promotion of tomato plants using endophytic bacteria isolated from Solanum elaeagnifolium stems. J. Phytopathol. 2016, 164, 811–824. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkila, O.; Fritze, H. Charcoal as a habitat for microbes and its effects on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar]
- Hale, L.; Luth, M.; Crowley, D. Biochar characteristics relate to its utility as an alternative soil inoculum carrier to peat and vermiculite. Soil Biol. Biochem. 2015, 81, 228–235. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Hashem, A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. 2015, 22, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Jabborova, D.; Wirth, S.; Alam, P.; Alyemeni, M.N.; Ahmad, P. Interactive Effects of Nutrients and Bradyrhizobium japonicum on the Growth and Root Architecture of Soybean (Glycine max L.). Front. Microbiol. 2018, 9, 1000. [Google Scholar]
- Cho, S.T.; Chang, H.H.; Egamberdieva, D.; Kamilova, F.; Lugtenberg, B.; Kuo, C.H. Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLoS ONE 2015, 10, e0140231. [Google Scholar]
- Egamberdieva, D.; Kucharova, Z. Selection for root colonising bacteria stimulating wheat growth in saline soils. Biol. Fert. Soils 2009, 45, 561–573. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Zech, W. Microbial response to charcoal amendments of highly weathered soils and Amazonian Dark Earths in Central Amazonia – Preliminary Results. In Amazonian Dark Earths: Explorations in Space and Time; Glaser, B., Woods, W.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Sangeetha, D. Survival of plant growth promoting bacterial inoculants indifferent carrier materials. Int. J. Pharm. Biol. Arch. 2012, 3, 170–178. [Google Scholar]
- Park, J.W.; Balaraju, K.; Kim, J.W.; Lee, S.W.; Park, K. Systemic resistance and growth promotion of chili pepper induced by an antibiotic producing Bacillus vallismortis strain BS07. Biol. Control 2013, 65, 246–257. [Google Scholar] [CrossRef]
- Parray, A.P.; Jan, S.; Kamili, A.N.; Qadri, R.A.; Egamberdieva, D.; Ahmad, P. Current perspectives on plant growth promoting rhizobacteria. Plant Growth Regul. 2016, 35, 877–902. [Google Scholar] [CrossRef]
- Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.A.; Malik, J.A.; Alharbi, R.I.; Egamberdieva, D. Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2017, 3, 37–44. [Google Scholar] [CrossRef] [Green Version]







| Material | DM (%FM) | Ash (%DM) | Ct (%DM) | Nt (%DM) | P (g/kg FM) | K (g/kg FM) | pH | EC |
|---|---|---|---|---|---|---|---|---|
| HTC-char | 47.39 | 3.19 | 64.55 | 2.09 | 1.02 | 3.58 | 5.25 | 0.30 |
| MBC | 92.85 | 18.42 | 75.16 | 1.65 | 5.26 | 31.12 | 9.89 | 3.08 |
| WBC | 55.09 | 16.64 | 77.62 | 0.72 | 1.24 | 7.8 | 9.35 | 1.71 |
| Sequences of Isolated Strains Deposited to GenBank | Closest Match among Bacteria (16S rRNA Genes) (GenBank) | ||||
|---|---|---|---|---|---|
| Strain | Length (bp) | Accession Number | Reference Strains | Accession Number | Percent Identity |
| L_1 | 1456 | MH636785 | Bacillus pumilus | NR_112637.1 | 99.25 |
| L_2 | 1437 | MH636786 | Pseudomonas putida | NR_113651.1 | 99.24 |
| L_3 | 1404 | MH636787 | Enterobacter cloacae | NR_044978.1 | 98.72 |
| L_4 | 1409 | MH636788 | Pseudomonas koreensis | NR_025228.1 | 99.15 |
| L_5 | 1406 | MH636789 | Pseudomonas thivervalensis | NR_024951.1 | 99.22 |
| L_6 | 1445 | MH636790 | Pseudomonas corrugata | NR_037135.1 | 99.03 |
| L_7 | 1436 | MH636791 | Pseudarthrobacter oxydans | NR_026236.1 | 99.23 |
| L_8 | 1485 | MH636792 | Stenotrophomonas pavanii | NR_116793.1 | 99.06 |
| L_9 | 1358 | MH636793 | Cellulosimicrobium cellulans | NR_115251.1 | 98.75 |
| Bacterial Isolates | Exo-Enzymes | Antagonistic Activity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCN production | Siderophore | IAA (µg/mL) | Protease | Cellulase | Chitinase | F.oxysporum | F.solani | F.culmorum | A.alternata | B.cinerea | |
| B. pumilus L1 | − | − | − | − | − | − | − | − | − | − | − |
| P. putida L2 | + | + | + | − | + | + | + | + | + | − | − |
| E. cloacae L3 | − | − | − | − | − | − | − | − | − | − | − |
| P. koreensis L4 | − | − | − | − | + | − | + | + | − | − | − |
| P. thivervalensis L5 | − | − | − | − | − | + | − | − | − | − | − |
| P. corrugata L6 | − | − | − | + | − | − | − | − | − | + | + |
| P. oxydans L7 | + | − | − | − | − | − | − | − | − | − | − |
| S. pavanii L8 | − | − | + | − | + | + | + | + | − | − | + |
| C. cellulans L9 | − | − | + | + | − | − | − | − | − | − | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egamberdieva, D.; Shurigin, V.; Alaylar, B.; Ma, H.; Müller, M.E.H.; Wirth, S.; Reckling, M.; Bellingrath-Kimura, S.D. The Effect of Biochars and Endophytic Bacteria on Growth and Root Rot Disease Incidence of Fusarium Infested Narrow-Leafed Lupin (Lupinus angustifolius L.). Microorganisms 2020, 8, 496. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8040496
Egamberdieva D, Shurigin V, Alaylar B, Ma H, Müller MEH, Wirth S, Reckling M, Bellingrath-Kimura SD. The Effect of Biochars and Endophytic Bacteria on Growth and Root Rot Disease Incidence of Fusarium Infested Narrow-Leafed Lupin (Lupinus angustifolius L.). Microorganisms. 2020; 8(4):496. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8040496
Chicago/Turabian StyleEgamberdieva, Dilfuza, Vyacheslav Shurigin, Burak Alaylar, Hua Ma, Marina E. H. Müller, Stephan Wirth, Moritz Reckling, and Sonoko Dorothea Bellingrath-Kimura. 2020. "The Effect of Biochars and Endophytic Bacteria on Growth and Root Rot Disease Incidence of Fusarium Infested Narrow-Leafed Lupin (Lupinus angustifolius L.)" Microorganisms 8, no. 4: 496. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8040496