Monitoring the Functionality and Stress Response of Yeast Cells Using Flow Cytometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Preparation and Sampling
2.2. Reagents and Chemicals
2.3. Cell Cycle Analysis
2.4. Intracellular Glycogen
2.5. Viability
2.6. Intracellular Neutral Lipids
2.7. Mitochondria Activity
2.8. Intracellular Reactive Oxygen Species
2.9. Degree of DNA Damage
2.10. Membrane Potential
2.11. Intracellular pH Value
2.12. Data Analysis
3. Results and Discussion
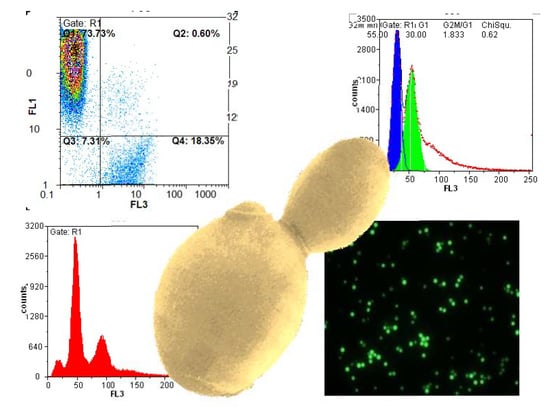
3.1. Interpretation of Flow Cytometry Data
3.2. Correlation of Physiological Yeast Data
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Hohmann, S.; Mager, W.H. Yeast Stress Responses; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Guerzoni, M.E.; Vernocchi, P.; Ndagijimana, M.; Gianotti, A.; Lanciotti, R. Generation of aroma compounds in sourdough: Effects of stress exposure and lactobacilli–yeasts interactions. Food Microbiol. 2007, 24, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, D.J.; Cliff, M.; van Vuuren, H.J.J. Impact of Yeast Strain on the Production of Acetic Acid, Glycerol, and the Sensory Attributes of Icewine. Am. J. Enol. Vitic. 2004, 55, 371–378. [Google Scholar]
- Bauer, F.; Pretorius, I.S. Yeast stress response and fermentation efficiency: How to survive the making of wine-a review. S. Afr. J. Enol. Vitic. 2000, 21, 27–51. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and Sluggish Fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Malherbe, S.; Bauer, F.F.; Du Toit, M. Understanding Problem Fermentations—A Review. S. Afr. J. Enol. Vitic. 2007, 28, 169–186. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.M. Yeast Physiology and Biotechnology; John Wiley & Sons: West Sussex, UK, 2000. [Google Scholar]
- Martínez-Muñoz, G.A.; Kane, P. Vacuolar and Plasma Membrane Proton Pumps Collaborate to Achieve Cytosolic pH Homeostasis in Yeast. J. Biol. Chem. 2008, 283, 20309–20319. [Google Scholar] [CrossRef] [Green Version]
- Imlay, J.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309. [Google Scholar] [CrossRef] [Green Version]
- Rambeck, W.; Simon, H. Decrease of Glycogen and Trehalose in Yeast during Starvation and during Ethanol Formation under the Influence of Propanol or Ethanol. Hoppe-Seyler’s Z. Für Physiol. Chem. 1972, 353, 1107–1110. [Google Scholar] [CrossRef]
- Hutter, K.-J.; Eipel, H.E. Flow Cytometric Determinations of Cellular Substances in Algae, Bacteria, Moulds and Yeasts. Antonie Van Leeuwenhoek 1978, 44, 269–282. [Google Scholar] [CrossRef]
- Dittrich, H.H.; Großmann, M. Mikrobiologie des Weines, 3rd ed.; Auflage, N.B., Ed.; Verlag Eugen Ulmer GmbH & Co.: Stuttgart, Germany, 2005. [Google Scholar]
- Lillie, S.H.; Pringle, J.R. Reserve Carbohydrate Metabolism in Saccharomyces cerevisiae: Responses to Nutrient Limitation. J. Bacteriol. 1980, 143, 1384–1394. [Google Scholar] [CrossRef] [Green Version]
- Dolezel, J. Flow cytometry principles and applications in mutation breeding. In Proceedings of the Fourteenth IAEA/FAO Interregional Training Course on Advances in Plant Mutation Techniques, Vienna, Austria; 1995; pp. 1–25. [Google Scholar]
- Sommer, S.; Wegmann-Herr, P.; Wacker, M.; Fischer, U. Influence of Lysozyme Addition on Hydroxycinnamic Acids and Volatile Phenols during Wine Fermentation. Fermentation 2018, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Longin, C.; Petitgonnet, C.; Guilloux-Benatier, M.; Rousseaux, S.; Alexandre, H. Application of flow cytometry to wine microorganisms. Food Microbiol. 2017, 62, 221–231. [Google Scholar] [CrossRef]
- Hutter, K.-J. Flow Cytometry—A New Tool for Direct Control of Fermentation Processes. J. Inst. Brew. 2002, 108, 48–51. [Google Scholar] [CrossRef]
- Hutter, K.-J. Flow Cytometric Determinations of Glycogen Content of Yeast During Fermentation. J. Inst. Brew. 2002, 108, 52–53. [Google Scholar] [CrossRef]
- Hutter, K.-J.; Eipel, H.E. DNA-Determination of Yeast by Flow Cytometry. FEMS Microbiol. Lett. 1978, 3, 35–38. [Google Scholar] [CrossRef]
- Hutter, K.-J.; Görtz, T.; Eipel, H.E. Different Stages of DNA-Synthesis During the Growth of Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1978, 3, 291–294. [Google Scholar] [CrossRef]
- Müller, S.; Hutter, K.-J.; Bley, T.; Petzold, L.; Babel, W. Dynamics of Yeast Cell States During Proliferation and Non-Proliferation Periods in a Brewing Reactor Monitored by Multidimensional Flow Cytometry. Bioprocess Eng. 1997, 17, 287–293. [Google Scholar] [CrossRef]
- Schwinn, M.; Durner, D.; Delgado, A.; Fischer, U. Distribution of Yeast Cells, Temperature, and Fermentation By-Products in White Wine Fermentations. Am. J. Enol. Vitic. 2019, 70, 339–350. [Google Scholar] [CrossRef]
- El-Diwany, A.I.; El-Abyad, M.S.; El-Refai, A.H.; Sallam, L.A.; Allam, R.F. Effect of some fermentation parameters on ethanol production from beet molasses by Saccharomyces cerevisiae Y-7. Bioresour. Technol. 1992, 42, 191–195. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssière, J.-L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Alexandre, H.; Rousseaux, I.; Charpentier, C. Relationship between ethanol tolerance, lipid composition and plasma membrane fluidity in Saccharomyces cerevisiae and Kloeckera apiculata. FEMS Microbiol. Lett. 1994, 124, 17–22. [Google Scholar] [CrossRef]
- Wilson, W.A.; Roach, P.J.; Montero, M.; Baroja-Fernández, E.; Muñoz, F.J.; Eydallin, G.; Viale, A.M.; Pozueta-Romero, J. Regulation of glycogen metabolism in yeast and bacteria. FEMS Microbiol. Rev. 2010, 34, 952–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsit, S.; Dequin, S. Diversity and adaptive evolution of Saccharomyces wine yeast: A review. FEMS Yeast Res. 2015, 15, fov067. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, S. Monitoring the Functionality and Stress Response of Yeast Cells Using Flow Cytometry. Microorganisms 2020, 8, 619. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8040619
Sommer S. Monitoring the Functionality and Stress Response of Yeast Cells Using Flow Cytometry. Microorganisms. 2020; 8(4):619. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8040619
Chicago/Turabian StyleSommer, Stephan. 2020. "Monitoring the Functionality and Stress Response of Yeast Cells Using Flow Cytometry" Microorganisms 8, no. 4: 619. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8040619