Entrapped Psychrotolerant Yeast Cells within Pine Sawdust for Low Temperature Wine Making: Impact on Wine Quality
Abstract
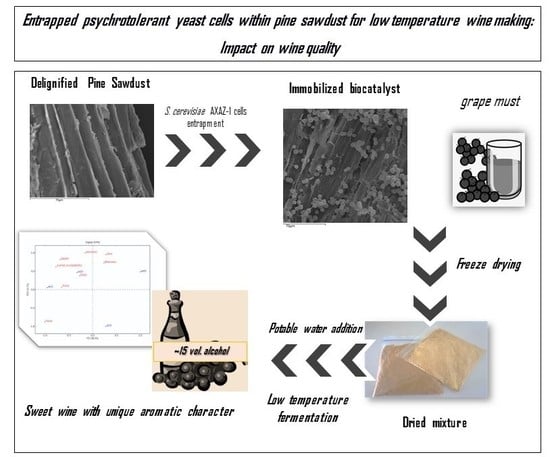
:1. Introduction
2. Materials and Methods
2.1. The Psychrotolerant Yeast Strain S. cerevisiae AXAZ-1
2.2. Tubular Cellulose as Immobilization Carrier
2.3. Scanning Electron Microscopy
2.4. Preparation of Fermentation Composite
2.5. High-Gravity Fermentation by Freeze-Dried Raw Materials
2.6. Analytical Methods
2.6.1. Sugar Analysis
2.6.2. Ethanol Determination
2.6.3. Solid Phase Microextraction (SPME) Gas Chromatography/Mass Spectrometry (GC/MS) Analysis
2.7. Sensory Evaluation by Principal Component Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Pine Sawdust Supported Biocatalyst Applied for Low Temperature Wine Making
3.2. Effect of Storage on Wines Aromatic Characteristics
3.3. Discrimination of Wine Flavor and Aroma Attributes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McGovern, P.; Jalabadze, M.; Batiuk, S.; Callahan, M.P.; Smith, K.E.; Hall, G.R.; Kvavadze, E.; Maghradze, D.; Rusishvili, N.; Bouby, L.; et al. Early Neolithic wine of Georgia in the South Caucasus. Proc. Natl. Acad. Sci. USA 2017, 114, E10309–E10318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, H.; Dooley, A.N.; Areshian, G.; Gasparyan, B.; Faull, K.F. Chemical evidence for wine production around 4000 BCE in the Late Chalcolithic Near Eastern highlands. J. Archaeol. Sci. 2011, 38, 977–984. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of clinical trials. Metabolism 2018, 83, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Prosapio, V.; Norton, I.; De Marco, I. Optimization of freeze-drying using a Life Cycle Assessment approach: Strawberries’ case study. J. Clean. Prod. 2017, 168, 1171–1179. [Google Scholar] [CrossRef]
- Berk, Z. Chapter 23-Freeze drying (lyophilization) and freeze concentration. In Food Process Engineering and Technology, 3rd ed.; Academic Press: New York, NY, USA, 2018; pp. 567–581. [Google Scholar] [CrossRef]
- Nicolosi, E.; Ferlito, F.; Amenta, M.; Russo, T.; Rapisarda, P. Changes in the quality and antioxidant components of minimally processed table grapes during storage. Sci. Hortic. 2018, 232, 175–183. [Google Scholar] [CrossRef]
- de Torres, C.; Schumacher, R.; Alañón, M.E.; Pérez-Coello, M.S.; Díaz-Maroto, M.C. Freeze-dried grape skins by-products to enhance the quality of white wines from neutral grape varieties. Food Res. Int. 2015, 69, 97–105. [Google Scholar] [CrossRef]
- Huang, K.; Fasahati, P.; Maravelias, C.T. System-Level Analysis of Lignin Valorization in Lignocellulosic Biorefineries. iScience 2020, 23, 100751. [Google Scholar] [CrossRef] [Green Version]
- Agouridis, N.; Kopsahelis, N.; Plessas, S.; Koutinas, A.A.; Kanellaki, M. Oenococcus oeni cells immobilized on delignified cellulosic material for malolactic fermentation of wine. Bioresour. Technol. 2008, 99, 9017–9020. [Google Scholar] [CrossRef]
- Golfinopoulos, A.; Soupioni, M.; Kanellaki, M.; Koutinas, A.A. pH Effect on Lactose Uptake Rate by Kefir Cells Immobilized on Delignified Cellulosic Material, Using 14C-Labelled Lactose during Whey Fermentation. J. Biotechnol. 2010, 150, 329. [Google Scholar] [CrossRef]
- Berbegal, C.; Polo, L.; García-Esparza, M.J.; Lizama, V.; Ferrer, S.; Pardo, I. Immobilisation of yeasts on oak chips or cellulose powder for use in bottle-fermented sparkling wine. Food Microbiol. 2019, 78, 25–37. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Sypsas, V.; Kandylis, P.; Michelis, A.; Bekatorou, A.; Kourkoutas, Y.; Kordulis, C.; Lycourghiotis, A.; Banat, I.M.; Nigam, P.; et al. Nano-tubular cellulose for bioprocess technology development. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gialleli, A.-I.; Ganatsios, V.; Terpou, A.; Kanellaki, M.; Bekatorou, A.; Koutinas, A.A.; Dimitrellou, D. Technological Development of Brewing in Domestic Refrigerator Using Freeze-Dried Raw Materials. Food Technol. Biotechnol. 2017, 55, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ganatsios, V.; Terpou, A.; Gialleli, A.-I.; Kanellaki, M.; Bekatorou, A.; Koutinas, A.A. A ready-to-use freeze-dried juice and immobilized yeast mixture for low temperature sour cherry (Prunus cerasus) wine making. Food Bioprod. Process. 2019, 117, 373–379. [Google Scholar] [CrossRef]
- Kallis, M.; Sideris, K.; Kopsahelis, N.; Bosnea, L.; Kourkoutas, Y.; Terpou, A.; Kanellaki, M. Pistacia terebinthus Resin as Yeast Immobilization Support for Alcoholic Fermentation. Foods 2019, 8, 127. [Google Scholar] [CrossRef] [Green Version]
- Comitini, F.; Capece, A.; Ciani, M.; Romano, P. New insights on the use of wine yeasts. Curr. Opin. Food Sci. 2017, 13, 44–49. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Effect on White Grape Must of Multiflora Bee Pollen Addition during the Alcoholic Fermentation Process. Molecules 2018, 23, 1321. [Google Scholar] [CrossRef] [Green Version]
- Cravero, M.C. Organic and biodynamic wines quality and characteristics: A review. Food Chem. 2019, 295, 334–340. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Evaluation of the use of multiflora bee pollen on the volatile compounds and sensorial profile of Palomino fino and Riesling white young wines. Food Res. Int. 2018, 105, 197–209. [Google Scholar] [CrossRef]
- Bardi, E.P.; Bakoyianis, V.; Koutinas, A.A.; Kanellaki, M. Room temperature and low temperature wine making using yeast immobilized on gluten pellets. Process Biochem. 1996, 31, 425–430. [Google Scholar] [CrossRef]
- García-Alcaraz, J.L.; Flor Montalvo, F.J.; Martínez Cámara, E.; Pérez de la Parte, M.M.; Jiménez-Macías, E.; Blanco-Fernández, J. Economic-environmental impact analysis of alternative systems for red wine ageing in re-used barrels. J. Clean. Prod. 2020, 244, 118783. [Google Scholar] [CrossRef]
- Martins, N.; Garcia, R.; Mendes, D.; Costa Freitas, A.M.; da Silva, M.G.; Cabrita, M.J. An ancient winemaking technology: Exploring the volatile composition of amphora wines. LWT 2018, 96, 288–295. [Google Scholar] [CrossRef]
- Fang, F.; Li, J.-M.; Zhang, P.; Tang, K.; Wang, W.; Pan, Q.-H.; Huang, W.-D. Effects of grape variety, harvest date, fermentation vessel and wine ageing on flavonoid concentration in red wines. Food Res. Int. 2008, 41, 53–60. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Kopsahelis, N.; Kokkali, V.; Terpou, A.; Kanellaki, M. Production of a novel probiotic yogurt by incorporation of L. casei enriched fresh apple pieces, dried raisins and wheat grains. Food Bioprod. Process. 2017, 102, 62–71. [Google Scholar] [CrossRef]
- Armani, M.; Morozova, K.; Scampicchio, M. Immobilization of Saccharomyces cerevisiae on nylon-6 nanofibrous membranes for grape juice fermentation. LWT 2019, 110, 360–364. [Google Scholar] [CrossRef]
- Mallouchos, A.; Komaitis, M.; Koutinas, A.; Kanellaki, M. Wine fermentations by immobilized and free cells at different temperatures. Effect of immobilization and temperature on volatile by-products. Food Chem. 2003, 80, 109–113. [Google Scholar] [CrossRef]
- Ganatsios, V.; Koutinas, A.A.; Bekatorou, A.; Panagopoulos, V.; Banat, I.M.; Terpou, A.; Kopsahelis, N. Porous cellulose as promoter of oil production by the oleaginous yeast Lipomyces starkeyi using mixed agroindustrial wastes. Bioresour. Technol. 2017, 244, 629–634. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Kanellaki, M.; Bekatorou, A. Low temperature brewing using cells immobilized on brewer’s spent grains. Food Chem. 2007, 104, 480–488. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Genisheva, Z.; Vilanova, M.; Mussatto, S.I.; Teixeira, J.A.; Oliveira, J.M. Consecutive alcoholic fermentations of white grape musts with yeasts immobilized on grape skins–Effect of biocatalyst storage and SO2 concentration on wine characteristics. LWT Food Sci. Technol. 2014, 59, 1114–1122. [Google Scholar] [CrossRef] [Green Version]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef] [Green Version]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [Green Version]
- Argiriou, T.; Kaliafas, A.; Psarianos, K.; Kanellaki, M.; Voliotis, S.; Koutinas, A.A. Psychrotolerant Saccharomyces cerevisiae strains after an adaptation treatment for low temperature wine making. Process Biochem. 1996, 31, 639–643. [Google Scholar] [CrossRef]
- Rodrigues, H.; Sáenz-Navajas, M.-P.; Franco-Luesma, E.; Valentin, D.; Fernández-Zurbano, P.; Ferreira, V.; De La Fuente Blanco, A.; Ballester, J. Sensory and chemical drivers of wine minerality aroma: An application to Chablis wines. Food Chem. 2017, 230, 553–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoina, V.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin for functional myzithra cheese manufacture. LWT Food Sci. Technol. 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Kadri, N.; Khettal, B.; Aid, Y.; Kherfellah, S.; Sobhi, W.; Barragan-Montero, V. Some physicochemical characteristics of pinus (Pinus halepensis Mill., Pinus pinea L., Pinus pinaster and Pinus canariensis) seeds from North Algeria, their lipid profiles and volatile contents. Food Chem. 2015, 188, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. 7-Fermentation. In Wine Science, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; pp. 332–417. [Google Scholar] [CrossRef]
- Galafassi, S.; Toscano, M.; Vigentini, I.; Piškur, J.; Compagno, C. Osmotic stress response in the wine yeast Dekkera bruxellensis. Food Microbiol. 2013, 36, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xu, H.; Feng, L.; Yu, Z.; Zhao, H.; Zhao, M. Fermentation performance of lager yeast in high gravity beer fermentations with different sugar supplementations. J. Biosci. Bioeng. 2016, 122, 583–588. [Google Scholar] [CrossRef]
- Lo, T.-M.; Teo, W.S.; Ling, H.; Chen, B.; Kang, A.; Chang, M.W. Microbial engineering strategies to improve cell viability for biochemical production. Biotechnol. Adv. 2013, 31, 903–914. [Google Scholar] [CrossRef]
- Terpou, A.; Dimopoulou, M.; Belka, A.; Kallithraka, S.; Nychas, G.-J.E.; Papanikolaou, S. Effect of Myclobutanil Pesticide on the Physiological Behavior of Two Newly Isolated Saccharomyces cerevisiae Strains during Very-High-Gravity Alcoholic Fermentation. Microorganisms 2019, 7, 666. [Google Scholar] [CrossRef]
- Pérez, D.; Assof, M.; Bolcato, E.; Sari, S.; Fanzone, M. Combined effect of temperature and ammonium addition on fermentation profile and volatile aroma composition of Torrontés Riojano wines. LWT Food Sci. Technol. 2018, 87, 488–497. [Google Scholar] [CrossRef]
- Sullivan, M.L.; Bradford, B.J. Viable cell yield from active dry yeast products and effects of storage temperature and diluent on yeast cell viability1. J. Dairy Sci. 2011, 94, 526–531. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.; Buglass, A.J.; Gook Lee, C. Aromatized Wines. In Handbook of Alcoholic Beverages: Technical, Analytical and Nutritional Aspects; Wiley: New York, NY, USA, 2010; Volume 1, pp. 436–446. [Google Scholar]
- Spanos, K.; Gaitanis, D.; Spanos, I. Resin production in natural Aleppo pine stands in northern Evia, Greece. Web Ecol. 2010, 10, 38–43. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Miró, P.; Serra-Cayuela, A.; Buxaderas, S.; López-Tamames, E. Assessment of the aroma profiles of low-alcohol beers using HS-SPME–GC-MS. Food Res. Int. 2014, 57, 196–202. [Google Scholar] [CrossRef]
- Wong, K.C.; Teng, Y.E. Volatile Components of Mimusops elengi L. Flowers. J. Essent. Oil Res. 1994, 6, 453–458. [Google Scholar] [CrossRef]
- Kandylis, P.; Vekiari, A.S.; Kanellaki, M.; Grati Kamoun, N.; Msallem, M.; Kourkoutas, Y. Comparative study of extra virgin olive oil flavor profile of Koroneiki variety (Olea europaea var. Microcarpa alba) cultivated in Greece and Tunisia during one period of harvesting. LWT Food Sci. Technol. 2011, 44, 1333–1341. [Google Scholar] [CrossRef]
- Kandylis, P.; Mantzari, A.; Koutinas, A.A.; Kookos, I.K. Modelling of low temperature wine-making, using immobilized cells. Food Chem. 2012, 133, 1341–1348. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.I.; Bosnea, L.; Kanellaki, M.; Koutinas, A.A.; Castro, G.R. Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT Food Sci. Technol. 2017, 79, 616–624. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Lopez-Tamames, E.; Buxaderas, S. Assessment of the volatile composition of juices of apricot, peach, and pear according to two pectolytic treatments. J. Agric. Food Chem. 2005, 53, 7837–7843. [Google Scholar] [CrossRef] [PubMed]
- Kandylis, P.; Manousi, M.E.; Bekatorou, A.; Koutinas, A.A. Freeze-dried wheat supported biocatalyst for low temperature wine making. LWT Food Sci. Technol. 2010, 43, 1485–1493. [Google Scholar] [CrossRef]
- Smadja, J.; Rondeau, P.; Sing Alain Shum, C. Volatile constituents of five Citrus Petitgrain essential oils from Reunion. Flavour Fragr. J. 2005, 20, 399–402. [Google Scholar] [CrossRef]
- Ratledge, C. 7-Lipids and Fatty Acids A2-ROSE, A.H. In Economic Microbiology: Primary Products of Metabolism; Academic Press: New York, NY, USA, 1978; pp. 263–302. [Google Scholar] [CrossRef]
- Schwab, W.; Fischer, T.; Wüst, M. Terpene glucoside production: Improved biocatalytic processes using glycosyltransferases. Eng. Life Sci. 2015, 15, 376–386. [Google Scholar] [CrossRef]
- Rothe, M. Volatile Compounds in Foods and Beverages. Herausgegeben von H. Maarse. 764 Seiten, zahlr. Abb. und Table Marcel Dekker, Inc., New York, Basel, Hong Kong 1991. Preis 172.50 $. Food Nahr. 2006, 35, 1080. [Google Scholar] [CrossRef]
- Fraile, P.; Garrido, J.; Ancín, C. Influence of a Saccharomyces cerevisiae Selected Strain in the Volatile Composition of Rosé Wines. Evolution during Fermentation. J. Agric. Food Chem. 2000, 48, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Hirst, M.B.; Richter, C.L. Review of Aroma Formation through Metabolic Pathways of Saccharomyces cerevisiae in Beverage Fermentations. Am. J. Enol. Vitic. 2016, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- López de Lerma, N.; Peinado, R.A. Use of two osmoethanol tolerant yeast strain to ferment must from Tempranillo dried grapes. Effect on wine composition. Int. J. Food Microbiol. 2011, 145, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Gambuti, A.; Piombino, P.; Moio, L. Sensory properties and aroma compounds of sweet Fiano wine. Food Chem. 2007, 103, 1228–1236. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science-Sensory Perception and Wine Assessment. In Wine Science, 4th ed.; Academic Press: San Diego, CA, USA, 2014; pp. 831–888. [Google Scholar] [CrossRef]
- Shinohara, T. Gas Chromatographic Analysis of Volatile Fatty Acids in Wines. Agric. Biol. Chem. 1985, 49, 2211–2212. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]





| Identified Compound | ID ** | KI ** | KIref ** | Content of Volatile Compounds in Samples (μg/L) | |||
|---|---|---|---|---|---|---|---|
| Wine 1 * | Wine 2 * | Wine 3 * | Wine 4 * | ||||
| Esters | |||||||
| Ethyl acetate | MS, KI | 886 | 894 a | 3692.8 | 3934.7 | 2389.7 | 940.9 |
| Ethyl butanoate | MS, KI | 1018 | 1040 e | 244.9 | 298.03 | 145.1 | 74.7 |
| Ethyl 2-methyl butanoate | MS, KI | 1036 | 1063 c | 234.5 | 180.5 | 200.6 | 190.6 |
| Ethyl pentanoate | MS, KI | 1054 | 1148 f | 110.3 | 125.6 | 95.4 | 88.5 |
| Isoamyl acetate | MS, KI | 1109 | 1123 e | 654.5 | 1566.3 | 390.1 | 745.7 |
| Ethyl hexanoate | MS, KI | 1212 | 1250 f | 2965.7 | 4809.6 | 9668.7 | 9887.1 |
| Hexyl acetate | MS, KI | 1253 | 1281 c | 334.2 | 255.9 | 613.12 | 867.5 |
| Ethyl octanoate | MS, KI | 1386 | 1421 f | 7980.6 | 11,359.3 | 4626.1 | 2257.7 |
| Ethyl decanoate | MS, KI | 1599 | 1635 e | 2696.2 | 5248.9 | 3417.1 | 4138.7 |
| Diethyl succinate | MS, KI | 1639 | 1679 h | 6882.7 | 6304.2 | 6072.6 | 4229.8 |
| Ethyl 9-decenoate | MS, KI | 1659 | 1692 h | 875.6 | 613.1 | 1088.5 | 1584.2 |
| Ethyl benzeneacetate | MS, KI | 1762 | 1809 e | 87.2 | 88.5 | 70.6 | 73.1 |
| 2-Phenylethyl acetate | MS, KI | 1796 | 1832 a | 884.1 | 2723.3 | 3489.2 | 2277.8 |
| Ethyl dodecanoate | MS, KI | 1821 | 1850 e | 133.8 | 102.4 | 164.5 | 197.8 |
| 2-Phenylethyl butanoate | MS, KI | 1954 | 1930 c | 304.2 | 337.9 | 287.1 | 231.4 |
| Isopropyl myristate | MS, KI | 2030 | 2041 e | 1204.0 | 2613.0 | 735.19 | 441.44 |
| Ethyl tetradecanoate | MS, KI | 2036 | 2056 c | 250.5 | 230.7 | 200.8 | 220.4 |
| Alcohols | |||||||
| 4-methyl 1-pentenol | MS, KI | 1253 | 1282 e | 39.5 | 40.2 | 28.7 | 29.4 |
| 3-penten-1-ol | MS, KI | 1310 | 1305 d | 184.5 | 194.1 | 170.5 | 105.6 |
| 1-Hexanol | MS, KI | 1331 | 1340 b | 590.0 | 1059.8 | 1093.4 | 745.5 |
| 3-Methyl 1-pentanol | MS, KI | 1353 | 1350 e | 131.5 | 122.5 | 130.5 | 110.4 |
| 2-ethyl 1-hexanol | MS, KI | 1445 | 1490 d | 2015.6 | 1805.4 | 1742.3 | 1889.7 |
| 1-Heptanol | MS, KI | 1463 | 1464 a | 112.3 | 144.2 | 115.7 | 110.8 |
| 1-Octanol | MS, KI | 1512 | 1536 f | 112.4 | 111.9 | 496.0 | 518.09 |
| 1,3- Butanediol | MS, KI | 1616 | 1590 e | 859.5 | nd | nd | nd |
| Phenylethyl alcohol | MS, KI | 1896 | 1938 a | 11,487.2 | 10,254.3 | 3078.2 | 1794.7 |
| Acids | |||||||
| Hexanoic acid | MS, KI | 1873 | 1851 f | 846 | nd | nd | nd |
| Octanoic acid | MS, KI | 2078 | 2111 a | 11,671.12 | 12,877.61 | 1257.12 | 2704.15 |
| n-Decanoic acid | MS, KI | 2293 | 2336 e | 6125.86 | 7235.31 | 5323.95 | 3199.69 |
| Carbonyl compounds | |||||||
| Nonanal | MS, KI | 1349 | 1395 f | 33.5 | nd | 35.2 | 40.5 |
| Furfural | MS, KI | 1426 | 1475 a | 350.6 | 360.2 | 343.5 | 393.3 |
| Decanal | MS, KI | 1457 | 1507 a | 190.2 | nd | nd | nd |
| Terpenoid compounds | |||||||
| D-Limonene | MS, KI | 1119 | 1189 b | 241.2 | 180.5 | 155.6 | 124.7 |
| Linalool | MS, KI | 1500 | 1500 i | 775.9 | 2369.2 | 1268.6 | 392.9 |
| α-Terpineol | MS, KI | 1665 | 1661 n | 850.5 | 976.5 | 737.2 | 700.6 |
| β-Citronellol | MS, KI | 1733 | 1711 h | 112.3 | 111.3 | 110.3 | 110.5 |
| Anethole | MS, KI | 1819 | 1843 e | 156.1 | nd | nd | nd |
| Geranyl acetone | MS, KI | 1841 | 1853 g | 663.6 | nd | nd | nd |
| Nerolidol | MS, KI | 2030 | 2053 c | 316.8 | nd | nd | nd |
| Farnesol | MS, KI | 2283 | 2343 e | 5277.6 | 4512.6 | 4156.3 | 4215.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpou, A.; Ganatsios, V.; Kanellaki, M.; Koutinas, A.A. Entrapped Psychrotolerant Yeast Cells within Pine Sawdust for Low Temperature Wine Making: Impact on Wine Quality. Microorganisms 2020, 8, 764. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8050764
Terpou A, Ganatsios V, Kanellaki M, Koutinas AA. Entrapped Psychrotolerant Yeast Cells within Pine Sawdust for Low Temperature Wine Making: Impact on Wine Quality. Microorganisms. 2020; 8(5):764. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8050764
Chicago/Turabian StyleTerpou, Antonia, Vassilios Ganatsios, Maria Kanellaki, and Athanasios A. Koutinas. 2020. "Entrapped Psychrotolerant Yeast Cells within Pine Sawdust for Low Temperature Wine Making: Impact on Wine Quality" Microorganisms 8, no. 5: 764. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8050764