3.1. IR and Raman Spectra of Prehnite Measured under Ambient Conditions
Table 1 summarizes the peaks derived from the ambient infrared spectra obtained by smoothing the spectra with a Savitsky–Golay function and then deconvoluting and fitting peaks with multiple Gaussian functions (using PeakFit [
13]). There is acontinuous band of modes extending from 128 to 574 cm
−1, followed by a gap, then a band of peaks between 755 to 1150 cm
−1, followed by another gap before peaks between 3450 to 3500 cm
−1 (O-H strecthing modes). The peak at 475(1) cm
−1 is largely associated with the internal vibrational modes of the MO
6 octahedra, and the bands between 3453–3490 cm
−1 are associated with O–H stretching vibrations. The peaks within the 976–1152 cm
−1 range of the spectra reflect the internal vibrational modes of the SiO
4 tetrahedra. These assignments are based on the analyses of the infrared and Raman spectra of relevant silicate minerals [
14,
15,
16,
17,
18]. However, the remainder of the vibrational bands in the spectra are complex and is comprised of multiple peaks that cannot be assigned unambiguously without a full lattice dynamical study, which is beyond the scope of the current contribution.
Table 1 also includes the peaks obtained from fitting the Raman spectra. There is a continuous band of modes extending from 112 to 605 cm
−1, then a gap before modes between 940 to 1100 cm
−1 (Si–O stretching modes), and then another gap before the modes between 3450 to 3500 cm
−1 (O–H stretching modes).
3.2. High Pressure Infrared Spectra of Prehnite
The far- and mid-IR spectra of prehnite recorded over the 1 bar to 20 GPa range are shown in
Figure 2. These plots show the evolution with pressure of the Ca and lattice vibrational modes, as well as modes associated with the translational motions of the octahedral and tetrahedra [
14,
15,
16]. Examination of the spectra reveals a red-shift of the peak at ca. 540 cm
−1, accompanied by suppression of the peak intensity. The changes in this peak are gradual, but appear to commence at ca. 6.0 GPa and be completed by 8.2 GPa. A comparison of the high pressure far-IR spectra of prehnite with those of layered silicates, such as chrysotile, antigorite, talc, clinochlore, and montmorillonite [
14,
15,
16] suggest that the peaks within the 400–700 cm
−1 range are associated with the translational motions of the polyhedra, the bending/stretching of the P–O–P (P = polyhedron) links, and the M–OH vibrations.
Consequently, the changes observed between 6.0 and 8.2 GPa in the far-IR spectra, as evidenced by the red-shift of the 540 cm
–1 peak, are likely to involve a subtle change in the relative orientations of the polyhedra that is insufficient to break the average crystal symmetry (P
ncm) of the structure [
9]. However, the mid-IR region of the high-pressure spectra associated with the O–H stretching modes (
Figure 2C) do not exhibit any significant changes in the 6–8 GPa pressure range. This is further emphasized in
Figure 2D, where the positions of the primary O–H vibration peaks are plotted as a function of pressure. No deviation of the curve is observable within this pressure range. Above 16 GPa, however, there is sudden red-shift, which is discussed below. In contrast to the IR data, examination of the corresponding peaks in the high-pressure Raman spectra between 6–8 GPa (
Section 3.3) indicate that there is a change in the chemical environment of the O–H group associated with this phase transition.
The far-IR results are consistent with the HP-XRD results, that indicate the prehnite structure begins to soften above 8.7 GPa, in which subtle but significant structural changes occur [
10]. The broad pressure range, and shift to lower pressure, reflected in the IR data relative to the HP-XRD data, may be due to the non-hydrostatic conditions under which these spectroscopic data were collected (e.g., [
11]). Furthermore, the HP-XRD experiments indicated that the transition involves movement/distortion of the tetrahedra, as evidenced by alterations in the O–T–O and T–O–T (T = Si or Al) angles. The far-IR data concur with this finding.
The infrared spectra of prehnite over the 700–1300 cm
−1 energy range are displayed in
Figure 2B. These spectra contain information pertaining to the internal vibrational modes of both the octahedra and the tetrahedra, as well as complex interactive polyhedral modes. It is immediately apparent that the evolution of these spectra with pressure is more intricate than observed for the spectra recorded below 700 cm
−1 (
Figure 2A). This may be indicative of the internal vibrational modes of the polyhedra, and the complex coupled motions of these units, being more sensitive to pressure than the lattice modes and Ca–O vibrations. No abrupt changes are observed in the peaks from 900–1300 cm
−1 over the 6–8 GPa pressure range. This may further suggest that the structural transition that occurs within this pressure range does not necessitate a change in the lengths, and hence strength, of the bonds within the polyhedra, a finding that is also consistent with the HP-XRD study [
10].
A more dramatic change in the far-IR spectra is observed above 11 GPa (
Figure 2A). The peaks in the high-pressure far-IR spectra display a dramatic change in shape (predominately broadening) and reduction in the intensity between 11.5 and 12.4 GPa. As these bands are principally associated with the lattice modes and polyhedral motions, these significant changes in the spectra are believed to be associated with the onset of the collapse of the prehnite framework. This framework collapse is fully reversible. Similar effects are seen in the IR spectra, shown in
Figure 2B. At ca. 11 GPa, the bands begin to lose structure, broaden and weaken in intensity, coinciding with the onset of the framework collapse. In contrast, the O–H vibrational modes seem insensitive to the initial stages of this major structural change, as the peaks associated with these modes do not exhibit any notable changes until ca. 16 GPa, at which point there is a noticeable red-shift of the principle peak associated with the O–H vibrations (
Figure 2D).
3.3. High Pressure Raman Spectra of Prehnite
The high-pressure Raman spectra for prehnite are presented in
Figure 3. Many of the features that are apparent in the spectra measured under ambient conditions (
Table 1) are obscured by the high background in the high-pressure spectra. Nonetheless, valuable information can still be gleaned, in particular from the evolution of the peak at ca. 520 cm
−1 with pressure. This peak either arises from the internal vibrations of the MO
6 polyhedra, or from the T–O–T bending modes. As the pressure is increased from 6.73 GPa to 12.68 GPa, the splitting of this peak is amplified, yet as the pressure is decreased, the degree of splitting reduces, and below ca. 8.6 GPa, the peak becomes a single entity. This is strongly suggestive of an alteration of the prehnite structure with pressure. Furthermore, the merging of the split peak into a single peak below ca. 8.6 GPa coincides with the phase transition that is known to occur in prehnite at this pressure, as evidenced by the high-pressure IR spectra described above and the HP-XRD study [
6]. As discussed above, the HP-XRD study of prehnite indicated that the structural phase transition at ca. 8.7 GPa is related to a distortion of the tetrahedra. Consequently, it is probable that the peak at ca. 520 cm
–1 is due to T–O–T bending motions, and that in the prehnite phase that exists above 8.6 GPa, there is an increase in the number of tetrahedra in symmetrically distinct environments, that results in the splitting of this peak in the high pressure spectra. The peaks in the 850–950 cm
−1 range of the spectra (
Figure 3A) involve the internal vibrations, predominately T–O–T stretching, of the SiO
4 and AlO
4 tetrahedra. Unfortunately, the low resolution of the data and the interference of the methanol/ethanol solvent peak at ca. 1050 cm
−1 prevent a conclusive evaluation of the peaks in this energy range.
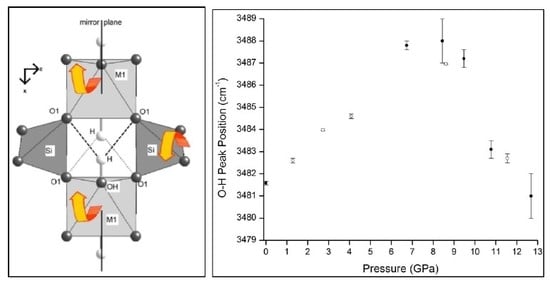
Figure 3B,C show the evolution of the O–H vibration band with pressure and the position of the principle peak of this band, respectively. As the pressure increases, the position of the O–H vibration peak is, as expected, blue-shifted. However, at ca. 8.6 GPa, there is a noticeable red-shift, as evidenced by the sudden downturn in the data plotted in
Figure 3C (closed circles); this provides further verification of a phase change occurring in the prehnite structure at ca. 8.6 GPa. Moreover, this phase transition is clearly accompanied by an alteration in the environment and weakening of the O–H bond. Such a change was not observed in the high-pressure mid-IR spectra of the sample (
Figure 2), and this is presumably due to the different selection rules associated with the two techniques. The shift to lower energy of the O–H bond vibration is indicative of the weakening of this bond, which may suggest that the hydrogen bonding in which this bond participates is strengthened by the structural alterations that occur during the phase transition. This is not altogether surprising, as this electrostatic interaction is between the –OH group situated at the apex of the MO6 octahedra and the T–O–M bridging oxygen located on the opposite side of the cavity [
2,
3]. Therefore, it is feasible that the deformation of the tetratahedra that is believed to drive the phase transition results in the distance between the bridging oxygen atom and the hydrogen atom being shortened, and consequently the O–H··O hydrogen bond is strengthened. The phase transition at observed 8.6 GPa is reversible. This is indicated by the return of the O–H peak to its low-pressure position, as the pressure on the structure is alleviated (
Figure 3C, open circles), and by the recombination below 8.6 GPa of the split peak at ca. 520 cm
–1 (
Figure 3B).