Impact of Paleosalinity, Paleoredox, Paleoproductivity/Preservation on the Organic Matter Enrichment in Black Shales from Triassic Turbidites of Semanggol Basin, Peninsular Malaysia
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
Location of Sample Collection
4. Results
4.1. Sedimentary Characteristics
4.2. Organic Geochemistry
4.3. Inorganic Geochemical Analysis
4.3.1. Major Oxides
Type of Clay
4.3.2. Trace Elements
4.3.3. Bioproductivity Proxies
4.3.4. Rare Earth Elements (REEs)
4.4. Mineralogical Compositions
5. Discussion
5.1. The Terrigenous Influx and Weathering Intensity
5.2. Paleoredox Conditions
5.3. Paleoclimatic Conditions
5.4. Paleosalinity
5.5. Paleoproductivity
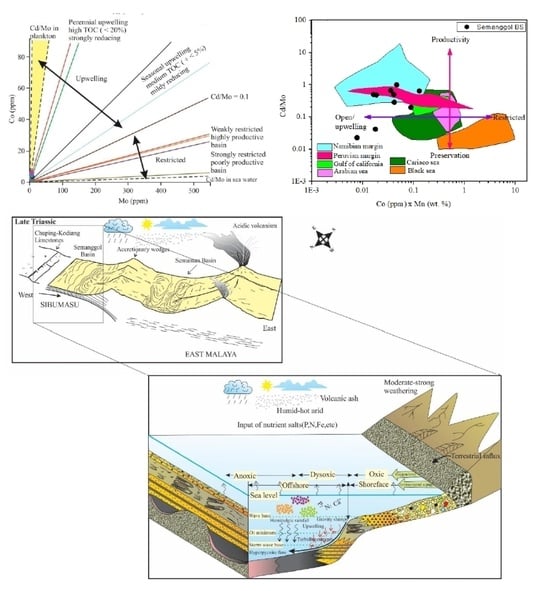
5.6. Geochemical Evidence of Ancient Upwelling
5.7. Impact of Geological Events on Depositional History
5.8. Organic Matter Enrichment
6. Conclusions
- (1)
- Mineralogical analysis reveals that dominant clay minerals in turbidite-associated black shale are kaolinite and illite, and non-clays like quartz with traces of feldspar and pyrite, and some heavy metals including zircon and titanium, are also detected. Th vs. K2O, and SiO2 vs. Al2O3, show that the dominant clay mineral types are illite and kaolinite with mixed clay layers, indicating a humid-to-temperate climate at mid-latitude.
- (2)
- The occurrence of type-III kerogens designate in the black shale and organic matter content is supposed to occur in mature to postmature phase.
- (3)
- CIA and CIW values indicate moderate to strong weathering conditions, anoxic-oxic oxygen levels, semi-arid to hot-arid climatic conditions, and a high sedimentary rate.
- (4)
- Paleosalinity of the basin is approximated based on Rb/K and Sr/Ba ratios, which suggest fresh to brackish and freshwater conditions. Intermittent volcanic nutrient fluxes caused an increase in salinity of water that led to the flourishing of marine organisms.
- (5)
- Paleo-upwelling conditions derived from various cross-plots (such as Al vs. Co × Mn; Al vs. CoEF × MnEF; Mo vs. Co; and Co × Mn vs. Cd/Mo) reveal that upwelling-controlled paleoproductivity and preservation have major control over the enrichment of organic matter, while the role of the sedimentary rate is comparatively less significant.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouma, A.H. Sedimentology of Some Flysch Deposits. A Graphic Approach to Facies Interpretation; Elsevier: Amsterdam, The Netherlands, 1962. [Google Scholar]
- Weimer, P.; Pettingill, H.S.; Nilsen, T.H. Global overview of deep-water exploration and production. Atlas Deep. Outcrops Am. Assoc. Pet. Geol. Stud. Geol. 2007, 56, 7–11. [Google Scholar]
- Xie, G.; Shen, Y.; Liu, S.; Hao, W. Trace and rare earth element (REE) characteristics of mudstones from Eocene Pinghu Formation and Oligocene Huagang Formation in Xihu Sag, East China Sea Basin: Implications for provenance, depositional conditions and paleoclimate. Mar. Pet. Geol. 2018, 92, 20–36. [Google Scholar] [CrossRef]
- Eltom, H.A.; Abdullatif, O.M.; Makkawi, M.H.; Eltoum, I.E.A. Rare earth element geochemistry of shallow carbonate outcropping strata in Saudi Arabia: Application for depositional environments prediction. Sediment. Geol. 2017, 348, 51–68. [Google Scholar]
- Rakocinski, M.; Zaton, M.; Marynowski, L.; Przemysław, G. Redox conditions, productivity, and volcanic input during deposition of uppermost Jurassic and Lower Cretaceous organic-rich siltstones in Spitsbergen, Norway. Cretac. Res. 2018, 89, 126–147. [Google Scholar] [CrossRef]
- Pisarzowska, A.; Rakociński, M.; Marynowski, L.; Szczerba, M.; Thoby, M.; Paszkowski, M.; Perri, M.C.; Spalletta, C.; Schönlaub, H.P.; Kowalik, N.; et al. Large environmental disturbances caused by magmatic activity during the Late Devonian Hangenberg Crisis. Glob. Planet. Chang. 2020, 190, 103155. [Google Scholar]
- Algeo, T.J.; Liu, J. A re-assessment of elemental proxies for paleoredox analysis. Chem. Geol. 2020, 540, 119549. [Google Scholar]
- Dypvik, H.; Harris, N.B. Geochemical facies analysis of fine-grained siliciclastics using Th/U, Zr/Rb and (Zr + Rb)/Sr ratios. Chem. Geol. 2001, 181, 131–146. [Google Scholar] [CrossRef]
- Szczepanik, P.; Witkowska, M.; Sawłowicz, Z. Geochemistry of Middle Jurassic mudstones (Kraków-Czestochowa area, southern Poland): Interpretation of the depositional redox conditions. Geol. Q. 2007, 51, 57–66. [Google Scholar]
- Racka, M.; Marynowski, L.; Filipiak, P.; Sobstel, M.; Pisarzowska, A.; Bond, D.P.G. Anoxic Annulata Events in the Late Famennian of the Holy Cross Mountains (Southern Poland): Geochemical and palaeontological record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 549–575. [Google Scholar] [CrossRef]
- Algeo, T.J.; Maynard, J.B. Trace-element behavior and redox facies in core shales of Upper Pennsylvanian Kansas-type cyclothems. Chem. Geol. 2004, 206, 289–318. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Sweere, T.; van den Boorn, S.; Dickson, A.J.; Reichart, G.J. Definition of new trace-metal proxies for the controls on organic matter enrichment in marine sediments based on Mn, Co, Mo and Cd concentrations. Chem. Geol. 2016, 441, 235–245. [Google Scholar] [CrossRef]
- Rimmer, S.M. Geochemical paleoredox indicators in Devonian—Mississippian black shales, Central Appalachian Basin (USA). Chem. Geol. 2004, 206, 373–391. [Google Scholar] [CrossRef]
- Metcalfe, L. Tectonic evolution of the Malay Peninsula. J. Asian Earth Sci. 2013, 76, 195–213. [Google Scholar] [CrossRef]
- Metcalfe, I. Tectonic framework and Phanerozoic evolution of Sundaland. Gondwana Res. 2011, 19, 3–21. [Google Scholar] [CrossRef]
- Metcalfe, I. The Bentong–Raub Suture Zone. J. Asian Earth Sci. 2000, 18, 691–712. [Google Scholar] [CrossRef]
- Hutchison, C.S. Ophiolite in Southeast Asia. Geol. Soc. Am. Bull. 1975, 86, 797–806. [Google Scholar] [CrossRef]
- Brayard, A.; Escarguel, G.; Bucher, H.; Monnet, C.; Brühwiler, T.; Goudemand, N.; Galfetti, T.; Guex, J. Good genes and good luck: Ammonoid diversity and the end-Permian mass extinction. Science 2009, 325, 1118–1121. [Google Scholar] [CrossRef]
- Abdullah, N.T. Mesozoic Stratigraphy. In Geology of Peninsular Malaysia; Hutchison, C.S., Tan, D.N.K., Eds.; University of Malaya, Geological Society of Malaysia: Kuala Lumpur, Malaysia, 2009; pp. 129–131. [Google Scholar]
- Tate, R.B.; Tan, D.N.K.; Ng, T.F. Geological Map of Peninsular Malaysia. Scale 1:1,000,000; Geological Society of Malaysia, University of Malaya: Kuala Lumpur, Malaysia, 2008. [Google Scholar]
- Alexander, J.B. Pre-Tertiary stratigraphic succession in Malaya. Nature 1959, 183, 230–231. [Google Scholar] [CrossRef]
- Teoh, L. Geology and mineral resources of the Sungai Tiang area, Kedah Darulaman. Geol. Surv. Malays. 1992, 5, 93. [Google Scholar]
- Courtier, D.B. The Geology and Mineral Resources of the Neighbourhood of the Kulim Area, Kedah; Geological Society of Malaysia: Kuala Lumpur, Malaysia, 1974. [Google Scholar]
- Burton, C.K. The palaeotectonic status of the Malay Peninsula. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1970, 7, 51–60. [Google Scholar] [CrossRef]
- Burton, C.K. The geology and mineral resources of the Bedung Area, Kedah, West Malaysia. Geol. Surv. Malays. Map Bull. 1988, 7, 103. [Google Scholar]
- Sashida, K.; Igo, H.; Adachi, S.; Koike, T.; Nakornsri, N.; Amnan, I.B.; Anlphoinnlahv, A. Occurrence of Paleozoic and Mesozoic radiolarians from Thailand and Malaysia and its geologic significance (Preliminery Report). News Osaka Micropaleontol. 1993, 9, 1–17. [Google Scholar]
- Sashida, K.; Adachi, S.; Igo, H.; Koike, T.; Amnan, I.B. 984 Middle and Late Permian radiolarians from the Semanggol Formation, Northwest Peninsular Malaysia. In Transactions and proceedings of the Paleontological Society of Japan; New Series; Palaeontological Society of Japan: Tokyo, Japan, 1995; pp. 43–58. [Google Scholar]
- Spiller, F.C.P.; Metcalfe, I. Late Palaeozoic radiolarians from the Bentong-Raub suture zone, and the Semanggol formation of Peninsular Malaysia—Initial results. J. Southeast. Asian Earth Sci. 1995, 11, 217–224. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tamura, M. The Triassic bivalvia of Malaysia, Thailand and adjacent areas. Palaeontol. Southeast. Asia 1984, 201–227. [Google Scholar]
- Katsuo, S. Middle and late Permian radiolarian from the semanggol formation, northwest Peninsula Malaysia. Trans. Proc. Paleontol Soc. Jpn. 1995, 177, 43–58. [Google Scholar]
- Hashim, A.S. Discovery of ammonoid (Agathiceras sp.) and crinoid stems in the Kenny Hill formation of Peninsular Malaysia, and its significance. War. Geol. 1985, 11, 205–212. [Google Scholar]
- Burton, C.K. Mesozoic. In Geology of the Malay Peninsula, West Malaysia and Singapore; Gobbett, D.J., Hutchison, C.S., Eds.; John Wiley-Interscience: New York, NY, USA, 1973; pp. 97–141. [Google Scholar]
- Jasin, B.; Jantan, A.; Abdullah, I.; Said, U.; Samsudin, A.R. The Semanggol Formation–lithostratigraphy of the Semanggol rocks in the light of latest concept in stratigraphic practice: A suggestion. War. Geol. Newslet. Geol. Soc. Malays. 1989, 15, 29. [Google Scholar]
- Ridd, M.F. A Middle Permian–Middle Triassic accretionary complex and a Late Triassic foredeep basin: Forerunners of an Indosinian (Late Triassic) thrust complex in the Thailand–Malaysia border area. J. Asian Earth Sci. 2013, 76, 99–114. [Google Scholar] [CrossRef]
- Jones, C.R. The Geology and Mineral Resources of Perlis, North Kedah and the Langkawi Islands; Geological Survey Headquarters: Kuala Lumpur, Malaysia, 1981. [Google Scholar]
- Jenner, G.A.; Longerich, H.P.; Jackson, S.E.; Fryer, B.J. ICP-MS—A powerful tool for high-precision trace-element analysis in Earth sciences: Evidence from analysis of selected USGS reference samples. Chem. Geol. 1990, 83, 133–148. [Google Scholar]
- Sajid, Z.; Ismail, M.S.; Tsegaband, H.; Hanif, T.; Ahmed, N. Sedimentary geology and geochemical approach to determine depositional environment of the Triassic turbidites bearing Semanggol Formation, NW Peninsular Malaysia. J. Nat. Gas. Geosci. 2020, 5, 207–226. [Google Scholar]
- Mukhopadhyay, P.K.; Wade, J.A.; Kruge, M.A. Organic facies and maturation of Jurassic/Cretaceous rocks, and possible oil-source rock correlation based on pyrolysis of asphaltenes, Scotian Basin, Canada. Org. Geochem. 1995, 22, 85–104. [Google Scholar]
- Espitalié, J.; Laporte, J.L.; Madec, M.; Marquis, F.; Leplat, P.; Paulet, J.; Boutefeu, A. Rapid method for source rocks characterization and for determination of petroleum potential and degree of evolution. Rev. L Inst. Fr. Du Pet. 1977, 32, 23–42. [Google Scholar]
- Sageman, B.B.; Lyons, T.W. Geochemistry fine-grained sediments and sedimentary rocks. In Treatise on Geochemistry; Mackenzie, F.T., Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 115–158. [Google Scholar]
- Parrish, J.T. Paleogeography of Corg-rich rocks and the preservation versus production controversy: Paleogeography, Paleoclimate, and Source Rocks. AAPG Studies Geol. 1995, 40, 1–20. [Google Scholar]
- Nesbitt, H.; Young, G.M. Early Proterozoic climates and plate motions inferred from major element chemistry of lutites. Nature 1982, 299, 715–717. [Google Scholar]
- Harnois, L. The CIW index: A new chemical index of weathering. Sediment. Geol. 1988, 55, 319–322. [Google Scholar]
- Kasanzu, C.; Maboko, M.A.H.; Manya, S. Geochemistry of fine-grained clastic sedimentary rocks of the Neoproterozoic Ikorongo Group, NE Tanzania: Implications for provenance and source rock weathering. Precambrian Res. 2008, 164, 201–213. [Google Scholar]
- Einsele, G. Sedimentary Basins: Evolution, Facies, and Sediment Budget; Springer Science & Business Media: Berlin, Germany, 2000; ISBN 354066193X. [Google Scholar]
- Diester-Haass, L.; Robert, C.; Chamley, H. Paleoproductivity and climate variations during Sapropel deposition in the eastern Mediterranean Sea. In Proceedings of the Ocean Drilling Program, Scientific Results; Robertson, A.H.F., Emeis, K.C., Richter, C., Camerlenghi, A., Eds.; Ocean Drilling Program: College Station, TX, USA, 1998; pp. 227–248. [Google Scholar]
- Chamley, H. Clay formation through weathering. In Clay Sedimentology; Springer: Berlin, Germany, 1989; pp. 21–50. [Google Scholar]
- Schlumberger. Log Interpretation Charts; Schlumberger Educational Services: Houston, TX, USA, 1989. [Google Scholar]
- Cullers, R.L.; Podkovyrov, V.N. Geochemistry of the Mesoproterozoic Lakhanda shales in southeastern Yakutia, Russia: Implications for mineralogical and provenance control, and recycling. Precambrian Res. 2000, 104, 77–93. [Google Scholar]
- Ross, D.J.K.; Bustin, R.M. Investigating the use of sedimentary geochemical proxies for paleoenvironment interpretation of thermally mature organic-rich strata: Examples from the Devonian-Mississippian shales, Western Canadian Sedimentary Basin. Chem. Geol. 2009, 260, 1–19. [Google Scholar]
- Calvert, S.E.; Pedersen, T.F. Geochemistry of Recent oxic and anoxic marine sediments: Implications for the geological record. Mar. Geol. 1993, 113, 67–88. [Google Scholar] [CrossRef]
- Wedepohl, K.H. Environmental influences on the chemical composition of shales and clays. Phys. Chem. Earth 1971, 8, 307–333. [Google Scholar] [CrossRef]
- Spears, D.A.; Zheng, Y. Geochemistry and origin of elements in some UK coals. Int. J. Coal Geol. 1999, 38, 161–179. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Zeng, Y.; Tan, F.; He, J. Geochemistry and origin of rare earth elements (REEs) in the Shengli River oil shale, northern Tibet, China. Geochemistry 2011, 71, 21–30. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, X.; Feng, X.; Song, C.; Wang, D.; Chen, W.; Zeng, S. Geochemical features of the black shales from the Wuyu Basin, southern Tibet: Implications for palaeoenvironment and palaeoclimate. Geol. J. 2017, 52, 282–297. [Google Scholar] [CrossRef]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Algeo, T.J.; Kuwahara, K.; Sano, H.; Bates, S.; Lyons, T.; Elswick, E.; Hinnov, L.; Ellwood, B.; Moser, J.; Maynard, J.B. Spatial variation in sediment fluxes, redox conditions, and productivity in the Permian–Triassic Panthalassic Ocean. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 308, 65–83. [Google Scholar] [CrossRef]
- Dymond, J.; Suess, E.; Lyle, M. Barium in deep-sea sediment: A geochemical proxy for paleoproductivity. Paleoceanography 1992, 7, 163–181. [Google Scholar] [CrossRef]
- Paytan, A.; Kastner, M. Benthic Ba fluxes in the central Equatorial Pacific, implications for the oceanic Ba cycle. Earth Planet. Sci. Lett. 1996, 142, 439–450. [Google Scholar] [CrossRef]
- Dean, W.E.; Gardner, J.V.; Piper, D.Z. Inorganic geochemical indicators of glacial-interglacial changes in productivity and anoxia on the California continental margin. Geochim. Cosmochim. Acta 1997, 61, 4507–4518. [Google Scholar] [CrossRef]
- Haskin, L.A.; Wildeman, T.R.; Haskin, M.A. An accurate procedure for the determination of the rare earths by neutron activation. J. Radioanal. Chem. 1968, 1, 337–348. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific Publications: Oxford, UK, 1985; pp. 46–92. [Google Scholar]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-fromations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Dai, S.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, J.; Fu, X.; Chen, W.; Feng, X.; Wang, D.; Song, C.; Wang, Z. Geochemical characteristics, redox conditions, and organic matter accumulation of marine oil shale from the Changliang Mountain area, northern Tibet, China. Mar. Pet. Geol. 2015, 64, 203–221. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Z.; Xu, S.; Sun, P.; Hu, X. Element response to the ancient lake information and its evolution history of argillaceous source rocks in the Lucaogou Formation in Sangonghe area of southern margin of Junggar Basin. J. Earth Sci. 2013, 24, 987–996. [Google Scholar] [CrossRef]
- Verdel, C.; van der Pluijm, B.A.; Niemi, N. Variation of illite/muscovite 40 Ar/39 Ar age spectra during progressive low-grade metamorphism: An example from the US Cordillera. Contrib. Mineral. Petrol. 2012, 164, 521–536. [Google Scholar] [CrossRef]
- Murphy, A.E.; Sageman, B.B.; Hollander, D.J.; Lyons, T.W.; Brett, C.E. Black shale deposition and faunal overturn in the Devonian Appalachian basin: Clastic starvation, seasonal water-column mixing, and efficient biolimiting nutrient recycling. Paleoceanography 2000, 15, 280–291. [Google Scholar] [CrossRef]
- Murray, R.W.; Leinen, M. Chemical transport to the seafloor of the equatorial Pacific Ocean across a latitudinal transect at 135 W: Tracking sedimentary major, trace, and rare earth element fluxes at the Equator and the Intertropical Convergence Zone. Geochim. Cosmochim. Acta 1993, 57, 4141–4163. [Google Scholar] [CrossRef]
- Ibach, L.E.J. Relationship between sedimentation rate and total organic carbon content in ancient marine sediments. Am. Assoc. Pet. Geol. Bull. 1982, 66, 170–188. [Google Scholar]
- Hatch, J.R.; Leventhal, J.S. Relationship between inferred redox potential of the depositional environment and geochemistry of the Upper Pennsylvanian (Missourian) Stark Shale Member of the Dennis Limestone, Wabaunsee County, Kansas, U.S.A. Chem. Geol. 1992, 99, 65–82. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A.C. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Pi, D.H.; Jiang, S.Y.; Luo, L.; Yang, J.H.; Ling, H.F. Depositional environments for stratiform witherite deposits in the Lower Cambrian black shale sequence of the Yangtze Platform, southern Qinling region, SW China: Evidence from redox-sensitive trace element geochemistry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 398, 125–131. [Google Scholar] [CrossRef]
- Mohamed, K.R.; Joeharry, N.A.M.; Leman, M.S.; Ali, C.A. The gua musang group: A newly proposed stratigraphic unit for the permo-triassic sequence of northern central belt, peninsular Malaysia. Bull. Geol. Soc. Malays. 2016, 62, 131–142. [Google Scholar] [CrossRef]
- Dodd, T.J.H.; Leslie, A.G.; Gillespie, M.R.; Dobbs, M.R.; Bide, T.P.; Kendall, R.S.; Kearsey, T.I.; Chiam, K.; Goay, M. Journal of Asian Earth Sciences Deep to shallow-marine sedimentology and impact of volcanism within the Middle Triassic Palaeo-Tethyan Semantan Basin, Singapore. J. Asian Earth Sci. 2020, 196, 104371. [Google Scholar] [CrossRef]
- Metcalfe, I. Tectonic evolution of Sundaland Tectonic evolution of Sundaland. Bull. Geol. Soc. Malays. 2017, 63, 27–60. [Google Scholar] [CrossRef]
- Zheng, Y.; Anderson, R.F.; van Geen, A.; Fleisher, M.Q. Remobilization of authigenic uranium in marine sediments by bioturbation. Geochim. Cosmochim. Acta 2002, 66, 1759–1772. [Google Scholar] [CrossRef]
- Zheng, Y.; Anderson, R.F.; Van Geen, A.; Kuwabara, J. Authigenic molybdenum formation in marine sediments: A link to pore water sulfide in the Santa Barbara Basin. Geochim. Cosmochim. Acta 2000, 64, 4165–4178. [Google Scholar] [CrossRef]
- Morford, J.L.; Russell, A.D.; Emerson, S. Trace metal evidence for changes in the redox environment associated with the transition from terrigenous clay to diatomaceous sediment, Saanich Inlet, BC. Mar. Geol. 2001, 174, 355–369. [Google Scholar] [CrossRef]
- Crusius, J.; Calvert, S.; Pedersen, T.; Sage, D. Rhenium and molybdenum enrichments in sediments as indicators of oxic, suboxic and sulfidic conditions of deposition. Earth Planet. Sci. Lett. 1996, 145, 65–78. [Google Scholar] [CrossRef]
- Algeo, T.J.; Rowe, H. Paleoceanographic applications of trace-metal concentration data. Chem. Geol. 2012, 324, 6–18. [Google Scholar] [CrossRef]
- Algeo, T.J.; Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum-uranium covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Cao, J.; Yang, R.; Yin, W.; Hu, G.; Bian, L.; Fu, X. Mechanism of Organic Matter Accumulation in Residual Bay Environments: The Early Cretaceous Qiangtang Basin, Tibet. Energy Fuels 2018, 32, 1024–1037. [Google Scholar] [CrossRef]
- Roy, D.K.; Roser, B.P. Climatic control on the composition of Carboniferous-Permian Gondwana sediments, Khalaspir basin, Bangladesh. Gondwana Res. 2013, 23, 1163–1171. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Chen, W.; Feng, X.; Wang, D.; Song, C.; Zeng, S. Elemental geochemistry of the early Jurassic black shales in the Qiangtang Basin, eastern Tethys: Constraints for palaeoenvironment conditions. Geol. J. 2016, 51, 443–454. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Li, J.; Huang, J.; Ge, D. Geochemical characteristics and depositional environment of the Middle Permian mudstones from central Qiangtang Basin, northern Tibet. Geol. J. 2016, 51, 560–571. [Google Scholar] [CrossRef]
- Feng, X.L.; Fu, X.G.; Tan, F.W.; Chen, W.B. Sedimentary environment characteristics of Upper Carboniferous Cameng Formation in Kongkong Chaka Area of Northern Qiangtang Basin, Tibet. Geoscience 2014, 28, 953–961. [Google Scholar]
- Cao, J.; Wu, M.; Chen, Y.; Hu, K.; Bian, L.; Wang, L.; Zhang, Y. Trace and rare earth element geochemistry of Jurassic mudstones in the northern Qaidam Basin, northwest China. Geochemistry 2012, 72, 245–252. [Google Scholar] [CrossRef]
- Yandoka, B.M.S.; Abdullah, W.H.; Abubakar, M.B.; Hakimi, M.H.; Adegoke, A.K. Geochemical characterisation of Early Cretaceous lacustrine sediments of Bima Formation, Yola Sub-basin, Northern Benue Trough, NE Nigeria: Organic matter input, preservation, paleoenvironment and palaeoclimatic conditions. Mar. Pet. Geol. 2015, 61, 82–94. [Google Scholar] [CrossRef]
- Beckmann, B.; Flögel, S.; Hofmann, P.; Schulz, M.; Wagner, T. Orbital forcing of Cretaceous river discharge in tropical Africa and ocean response. Nature 2005, 437, 241–244. [Google Scholar] [CrossRef]
- Blake, J.M.; Peters, S.C.; Johannesson, K.H. Application of REE geochemical signatures for Mesozoic sediment provenance to the Gettysburg Basin, Pennsylvania. Sediment. Geol. 2017, 349, 103–111. [Google Scholar] [CrossRef]
- Rainer, T.; Herlec, U.; Rantitsch, G.; Sachsenhofer, R.F.; Vrabec, M. Organic matter maturation vs clay mineralogy: A comparison for Carboniferous to Eocene sediments from the Alpine-Dinaride junction (Slovenia, Austria). Geologija 2002, 45, 513–518. [Google Scholar] [CrossRef]
- Frey, R.W.; Seilacher, A. Uniformity in marine invertebrate ichnology. Lethaia 1980, 13, 183–207. [Google Scholar] [CrossRef]
- Kübler, B.; Jaboyedoff, M. Illite crystallinity. Comptes Rendus L’académie Des. Sci. Iia-Earth Planet. Sci. 2000, 331, 75–89. [Google Scholar]
- Dellisanti, F.; Pini, G.A.; Baudin, F. Use of T max as a thermal maturity indicator in orogenic successions and comparison with clay mineral evolution. Clay Miner. 2010, 45, 115–130. [Google Scholar] [CrossRef]
- Ramirez-Caro, D. Rare Earth Elements (REE) as Geochemical Clues to Reconstruct Hydrocarbon Generation History. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2013. [Google Scholar]
- Campbell, F.A.; Williams, G.D. Chemical composition of shales of Mannville group (lower Cretaceous) of central Alberta, Canada. Am. Assoc. Pet. Geol. Bull. 1965, 49, 81–87. [Google Scholar]
- Zhen, Z.; Li, W.; Xu, L.; Wang, C.; Zhao, L. Characteristics of palaeosalinity and palaeoredox records in sediment from Dali Lake: Climate change in North China from 0 to 2100 cal BP. Quat. Geochronol. 2020, 60, 101104. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Sun, P.; Li, Y.; George, S.C. Sedimentary basin evolution, gravity flows, volcanism, and their impacts on the formation of the Lower Cretaceous oil shales in the Chaoyang Basin, northeastern China. Mar. Petroleum Geol. 2020, 119, 104472. [Google Scholar] [CrossRef]
- Nara, F.; Tani, Y.; Soma, Y.; Soma, M.; Naraoka, H.; Watanabe, T.; Horiuchi, K.; Kawai, T.; Oda, T.; Nakamura, T. Response of phytoplankton productivity to climate change recorded by sedimentary photosynthetic pigments in Lake Hovsgol (Mongolia) for the last 23,000 years. Quat. Int. 2005, 136, 71–81. [Google Scholar] [CrossRef]
- Wei, H.; Chen, D.; Wang, J.; Yu, H.; Tucker, M.E. Organic accumulation in the lower Chihsia Formation (Middle Permian) of South China: Constraints from pyrite morphology and multiple geochemical proxies. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 353–355, 73–86. [Google Scholar] [CrossRef]
- Dehairs, F.; Chesselet, R.; Jedwab, J. Discrete suspended particles of barite and the barium cycle in the open ocean. Earth Planet. Sci. Lett. 1980, 49, 528–550. [Google Scholar] [CrossRef]
- Stein, M.; Föllmi, K.B.; Westermann, S.; Godet, A.; Adatte, T.; Matera, V.; Fleitmann, D.; Berner, Z. Progressive palaeoenvironmental change during the late Barremian–early Aptian as prelude to Oceanic Anoxic Event 1a: Evidence from the Gorgo a Cerbara section (Umbria-Marche basin, central Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 302, 396–406. [Google Scholar] [CrossRef]
- Schoepfer, S.D.; Shen, J.; Wei, H.; Tyson, R.V.; Ingall, E.; Algeo, T.J. Total organic carbon, organic phosphorus, and biogenic barium fluxes as proxies for paleomarine productivity. Earth-Sci. Rev. 2015, 149, 23–52. [Google Scholar] [CrossRef]
- Kraal, P. Redox-dependent phosphorus burial in modern and ancient marine sediments. In Geologica Ultraiectina; Faculteit Geowetenschappen, Departement Aardwetenschappen: Utrecht, The Netherlands, 2011. [Google Scholar]
- Ingall, E.D.; Bustin, R.M.; Van Cappellen, P. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochim. Cosmochim. Acta 1993, 57, 303–316. [Google Scholar] [CrossRef]
- Ingall, E.; Kolowith, L.; Lyons, T.; Hurtgen, M. Sediment carbon, nitrogen and phosphorus cycling in an anoxic fjord, Effingham Inlet, British Columbia. Am. J. Sci. 2005, 305, 240–258. [Google Scholar] [CrossRef]
- Brumsack, H.-J. The trace metal content of recent organic carbon-rich sediments: Implications for Cretaceous black shale formation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 344–361. [Google Scholar] [CrossRef]
- Goldhammer, T.; Brüchert, V.; Ferdelman, T.G.; Zabel, M. Microbial sequestration of phosphorus in anoxic upwelling sediments. Nat. Geosci. 2010, 3, 557–561. [Google Scholar] [CrossRef]
- Liew, J.E.; Antonio, J.; Vintaned, G. Lithostratigraphy and palaeontology of the ‘black shale’ facies of the semanggol formation (chert unit) at bukit merah (perak, peninsular malaysia). Platf. J. Eng. 2019, 3, 33–45. [Google Scholar]
- Hutchison, C.S.; Tan, D.N.K. Geology of Peninsular Malaysia; University of Malaya: Kuala Lumpur, Malaysia; The Geological Society: London, UK, 2009; ISBN 9834429665. [Google Scholar]
- Kobayashi, T.; Burton, C.K.; Tokuyama, A.; Yin, E.H. The Vaonella and Halob~ a facies of the Thai-Malay peninsula compared with those of Japan: Geol. Paleo. Southeast. Asia 1966, 3, 98–122. [Google Scholar]
- Scholz, F.; Hensen, C.; Noffke, A.; Rohde, A.; Liebetrau, V.; Wallmann, K. Early diagenesis of redox-sensitive trace metals in the Peru upwelling area–response to ENSO-related oxygen fluctuations in the water column. Geochim. Cosmochim. Acta 2011, 75, 7257–7276. [Google Scholar] [CrossRef]
- Böning, P.; Brumsack, H.-J.; Böttcher, M.E.; Schnetger, B.; Kriete, C.; Kallmeyer, J.; Borchers, S.L. Geochemistry of Peruvian near-surface sediments. Geochim. Cosmochim. Acta 2004, 68, 4429–4451. [Google Scholar] [CrossRef]
- Mclennan, S.M.; Hemming, S.; Mcdaniel, D.K.; Hanson, G.N. Geochemical Approaches to Sedimentation, Provenance, and Tectonics. Geol. Soc. Am. Spec. Pap. 1993, 284, 21–40. [Google Scholar]
- Metcalfe, I.; Crowley, J.L. Upper Permian and Lower Triassic conodonts, high-precision U-Pb zircon ages and the Permian-Triassic boundary in the Malay Peninsula. J. Asian Earth Sci. 2020, 104403. [Google Scholar] [CrossRef]
- Dodd, T.J.H.; Gillespie, M.R.; Leslie, A.G.; Kearsey, T.I.; Kendall, R.S.; Bide, T.P.; Dobbs, M.R.; Millar, I.L.; Kim, M.; Lee, W.; et al. Journal of Asian Earth Sciences Paleozoic to Cenozoic sedimentary bedrock geology and lithostratigraphy of Singapore. J. Asian Earth Sci. 2019, 180, 103878. [Google Scholar] [CrossRef]
- Olgun, N.; Duggen, S.; Andronico, D.; Kutterolf, S.; Croot, P.L.; Giammanco, S.; Censi, P.; Randazzo, L. Possible impacts of volcanic ash emissions of Mount Etna on the primary productivity in the oligotrophic Mediterranean Sea: Results from nutrient-release experiments in seawater. Mar. Chem. 2013, 152, 32–42. [Google Scholar] [CrossRef]
- Xie, S.; Pancost, R.D.; Wang, Y.; Yang, H.; Wignall, P.B.; Luo, G.; Jia, C.; Chen, L. Cyanobacterial blooms tied to volcanism during the 5 my Permo-Triassic biotic crisis. Geology 2010, 38, 447–450. [Google Scholar] [CrossRef]
- Langmann, B.; Zaksek, K.; Hort, M.; Duggen, S. Volcanic ash as fertiliser for the surface ocean. Atmos. Chem. Phys. 2010, 10, 3891–3899. [Google Scholar] [CrossRef]
- Duggen, S.; Croot, P.; Schacht, U.; Hoffmann, L. Subduction zone volcanic ash can fertilize the surface ocean and stimulate phytoplankton growth: Evidence from biogeochemical experiments and satellite data. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Smith, D.B.; Zielinski, R.A.; Rose, W.I., Jr.; Huebert, B.J. Water-soluble material on aerosols collected within volcanic eruption clouds. J. Geophys. Res. Ocean. 1982, 87, 4963–4972. [Google Scholar] [CrossRef]
- Arthur, M.A.; Sageman, B.B. Deposi tional Mechanisms and Environments of Ancient Deposits. Annu. Rev. Earth Planet. Sci. 1994, 22, 499–551. [Google Scholar] [CrossRef]
- Demaison, G.J.; Moore, G.T. Anoxic environments and oil source bed genesis. Am. Assoc. Pet. Geol. Bull. 1980, 64, 1179–1209. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, S.; Lu, Y.; Xu, S.; Shu, Y.; Wang, Y. Productivity or preservation? The factors controlling the organic matter accumulation in the late Katian through Hirnantian Wufeng organic-rich shale, South China. Mar. Pet. Geol. 2019, 109, 22–35. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, J.; Tang, X.; Huo, Z.; Han, S.; Lang, Y.; Zheng, Y.; Li, X.; Liu, T. Elemental geochemical evidence for depositional conditions and organic matter enrichment of black rock series strata in an inter-platform basin: The lower carboniferous datang formation, Southern Guizhou, Southwest China. Minerals 2018, 8, 509. [Google Scholar] [CrossRef]
- Wignall, P.B. Black Shales; Oxford University Press: Oxford, UK, 1994; ISBN 0198540388. [Google Scholar]
- Vine, J.D.; Tourtel, E.B. Geochemistry of black shale deposits—A summary report. Econ. Geol. 1970, 65, 253–272. [Google Scholar] [CrossRef]
- Algeo, T.J.; Lyons, T.W. Mo–total organic carbon covariation in modern anoxic marine environments: Implications for analysis of paleoredox and paleohydrographic conditions. Paleoceanography 2006, 21. [Google Scholar] [CrossRef]
- Tenger; Liu, W.; Xu, Y.; Chen, J.; Hu, K.; Gao, C. Comprehensive geochemical identification of highly evolved marine hydrocarbon source rocks: Organic matter, paleoenvironment and development of effective hydrocarbon source rocks. Chin. J. Geochem. 2006, 25, 333–340. [Google Scholar] [CrossRef]
- Fu, X.; Tan, F.; Feng, X.; Wang, D.; Chen, W.; Song, C.; Zeng, S. Early Jurassic anoxic conditions and organic accumulation in the eastern Tethys. Int. Geol. Rev. 2014, 56, 1450–1465. [Google Scholar] [CrossRef]
- Bradley, W.H.; Eugster, H.P. Geochemistry and Paleolimnology of the Trona Deposits and Associated Authigenic Minerals of the Green River Formation of Wyoming; Geological Survey Professional Paper; US Government Printing Office: Washington, DC, USA, 1969. [Google Scholar]
- Desborough, G.A. A biogenic-chemical stratified lake model for the origin of oil shale of the Green River Formation: An alternative to the playa-lake model. Geol. Soc. Am. Bull. 1978, 89, 961–971. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Pevear, D.R.; Hill, R.J. Mineral surface control of organic carbon in black shale. Science 2002, 295, 657–660. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Z.; Bechtel, A.; Strobl, S.A.I.; Sun, P. Tectonic and climate control of oil shale deposition in the Upper Cretaceous Qingshankou Formation (Songliao Basin, NE China). Int. J. Earth Sci. 2013, 102, 1717–1734. [Google Scholar] [CrossRef]














| Facies Code | Description | Process Interpretation | Sediment Transport and Depositional Process | Significant Changes | |
|---|---|---|---|---|---|
| Facies 1 (F1) | Clast-supported conglomerate | Interpreted as lower portions of high-concentrated, coarse-grained turbidity current deposits that eroded and incorporated ripped-up mud-clasts during transportation | Massive: “Freezing” on decreasing bottom slopes due to intergranular friction and cohesion | Increased rate of density/energy, deposition, bed amalgamation, and fluidization |  Increased flow maturity, mud content and tractional |
| Facies 2 (F2) | Pebbly sandstone | Deposition by sandy turbidity currents of high-density | Massive: Rapid collective grain deposition of a pebble–sand mixture due to increased intergranular friction as the flows decelerate | 
 | |
| Facies 3 (F3) | Thick bedded sandstone | Massive SS lacking internal stratification indicating a sudden energy decrease in turbidity currents and rapid deposition of sediments from high-density flow. The absence of sedimentary structures reflects the rapid deposition of sediments | Deposition of massive sandstone may be attributable to non-turbulent liquefied or grain flows | ||
| Facies 4 (F4) | Massive sandstone with large Floating clasts. | Outsized clasts/blocks are interpreted as large debris falls from the walls of basins and transported by debris or sandy turbidity currents caused by tectonic activity/faulting or rafting on top of slumps | Deposition by debris or sandy turbidity currents | ||
| Facies 5 (F5) | Rhythmite or heterolithic interbedded sandstone, siltstone and mudstone/shale (rhythmite) facies | Shows its deposition by mature and highly turbulent low-density turbidity flows | Most of these sandstone–mudstone couplets were formed during the waning, tractional phase of turbidity current flow, followed by the suspension settling of sand and a combination of tractional reworking of suspension-fed silt and clay material and low rates of suspension fall-out | ||
| Facies 6 (F6) | Typical bouma facies Tabcd | Has resulted from gradual single waning low-density turbidity flow followed by wider tractional modification at late stages and sediment fallout from suspension | Low-density turbidity current and/or suspension fallout from a waning flow density | ||
| Facies 7(F7) | Major slump/mass transport deposit (MTD) | Mudstone-rich, disorganized, reflected to be developed by mass transport (which includes plastic slides, muddy debris flows, and slope failure) | Cessation of movement on decreasing bottom slopes because gravity forces no longer exceed or balance basal and internal friction | ||
| Facies 8(F8) | Very thin-bedded “zebra-striped” mudstones | Deposition by mature, lower-density turbidity current with tractional modification and pulsatory in nature from a hemi-pelagic setting | Waning turbulent flow deposited from suspension clouds of low-density flow |  Increased occurrence of low-density muddy and sandy turbidity currents | |
| Facies 9(F9) | Thin-medium bedded, finely laminated dark grey to black mudstone | Mudstone is deposited by (1) suspension fallout from the water column containing particles of hemi-pelagic mud, and (2) low-energy turbidity currents having muddy material | Load cast: Rapid suspension fallout of very fine sand/silt. Relatively dilute, well-stratified and fully turbulent flows | ||
| Location | Sample | TOC (wt.%) | Tmax (°C) | S1 (ppm) | S2 (ppm) | S3 (ppm) | HI (mg/g TOC) | OI (mg/g TOC) | PI | S1 + S2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Gunung Semanggol | 1 | 0.55 | 502 | 30 | 50 | 70 | 9 | 13 | 0.38 | 80 |
| 2 | 9.2 | 560 | 25 | 50 | 870 | 3 | 9 | 0.21 | 75 | |
| 3 | 11.71 | 611 | 20 | 60 | 910 | 1 | 8 | 0.25 | 80 | |
| 4 | 0.21 | 452 | 70 | 60 | 100 | 29 | 48 | 0.54 | 70 | |
| 5 | 0.48 | 490 | 40 | 30 | 170 | 8 | 21 | 0.33 | 70 | |
| Bukit Merah | 6 | 0.36 | 313 | 30 | 40 | 120 | 11 | 33 | 0.43 | 40 |
| 7 | 1.12 | 367 | 20 | 20 | 121 | 2 | 11 | 0.49 | 80 | |
| 8 | 2.57 | 359 | 30 | 50 | 180 | 2 | 7 | 0.38 | 70 | |
| Nami | 9 | 0.55 | 510 | 30 | 40 | 90 | 9 | 11 | 0.37 | 170 |
| 10 | 3.67 | 608 | 50 | 120 | 220 | 3 | 6 | 0.29 | 130 |
| Formation | Semanggol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Section | Gunung Semanggol | Bukit Merah | Nami | |||||||
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| SiO2 | 71.64 | 70.7 | 66.93 | 69.8 | 75.96 | 70.1 | 75.6 | 72.3 | 69.18 | 71.2 |
| Al2O3 | 15.8 | 17.2 | 20.14 | 17.89 | 11.99 | 14.4 | 12.51 | 13.31 | 18.13 | 15.3 |
| Fe2O3 | 1.22 | 1.11 | 1.23 | 1.08 | 1.12 | 1.39 | 1.46 | 1.44 | 1.54 | 1.23 |
| MgO | 0.88 | 0.73 | 0.7 | 0.82 | 0.5 | 0.43 | 0.52 | 0.69 | 0.7 | 0.57 |
| CaO | 0.02 | 0.03 | 0.01 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 |
| Na2O | 0.08 | 0.06 | 0.09 | 0.05 | 0.06 | 0.04 | 0.07 | 0.06 | 0.07 | 0.01 |
| K2O | 3.74 | 3.71 | 4.25 | 3.02 | 3.04 | 3.21 | 3.29 | 4.54 | 4.68 | 3.69 |
| TiO2 | 0.77 | 0.89 | 0.94 | 0.91 | 0.52 | 0.54 | 0.64 | 0.56 | 0.83 | 0.76 |
| P2O5 | 0.06 | 0.05 | 0.04 | 0.01 | 0.03 | 0.1 | 0.02 | 0.01 | 0.04 | 0.02 |
| MnO | 0.01 | 0.04 | 0.02 | 0.01 | 0.01 | 0.05 | 0.02 | 0.01 | 0.01 | 0.02 |
| LOI | 5.6 | 5.33 | 5.4 | 5.1 | 6.6 | 6.3 | 5.7 | 5.6 | 4.6 | 5.1 |
| TOC (wt.%) | 0.54 | 9.2 | 11.69 | 0.21 | 0.48 | 0.36 | 1.12 | 2.57 | 0.55 | 3.67 |
| CIA | 80.45 | 81.90 | 82.24 | 85.27 | 79.40 | 81.54 | 78.73 | 74.23 | 79.17 | 80.40 |
| CIW | 99.37 | 99.48 | 99.51 | 99.61 | 99.42 | 99.65 | 99.29 | 99.40 | 99.51 | 99.74 |
| C-Values | 0.39 | 0.41 | 0.73 | 0.56 | 0.20 | 0.28 | 0.31 | 0.40 | 0.35 | 0.40 |
| Al/Na | 140.90 | 204.51 | 159.64 | 255.26 | 142.56 | 256.83 | 127.50 | 158.26 | 184.77 | 148 |
| Formation | Semanggol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Section | Gunung Semanggol | Bukit Merah | Nami | |||||||
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| La | 57.6 | 56.1 | 74.4 | 77.3 | 35.8 | 33.7 | 81.9 | 66.7 | 38.9 | 31.4 |
| Sc | 15 | 14 | 17 | 16 | 12 | 12 | 15 | 14 | 9.0 | 11 |
| Th | 1.3 | 1.1 | 1.4 | 1.0 | 0.8 | 0.9 | 1.2 | 1.3 | 1.0 | 1.1 |
| Hf | 5.9 | 6.2 | 8 | 7.1 | 7.3 | 6.3 | 5.4 | 5.7 | 9.2 | 8.3 |
| Co | 5.3 | 4.2 | 3.0 | 2.1 | 1.0 | 2.3 | 2.7 | 2.4 | 2.3 | 2.3 |
| Zr | 222.4 | 215.1 | 268.6 | 222.8 | 271.7 | 217.1 | 182.4 | 171.1 | 351.9 | 322.1 |
| Cr | 68.4 | 615.8 | 88.9 | 75.3 | 47.9 | 54.7 | 13.7 | 6.8 | 82.1 | 75.3 |
| Sr | 35.9 | 33.2 | 33.7 | 31.7 | 32.5 | 29.5 | 31.3 | 44.1 | 49 | 37.1 |
| Cu | 23.8 | 19.2 | 1.0 | 3.1 | 2.1 | 2.6 | 36.1 | 32.5 | 7.8 | 6.5 |
| Ga | 20.9 | 19.2 | 21.1 | 13.2 | 12.6 | 19.2 | 21.7 | 17.1 | 16.2 | 15.3 |
| Rb | 175.5 | 166 | 165.5 | 144.2 | 130.9 | 171.1 | 183.2 | 154.3 | 144 | 131.2 |
| Ni | 23 | 19 | 20 | 17 | 20 | 22 | 29 | 21 | 20 | 27 |
| U | 3.5 | 3.3 | 3.7 | 3.1 | 3.2 | 2.9 | 3.9 | 3.2 | 2.8 | 2.3 |
| V | 123 | 131 | 145 | 111 | 106 | 144 | 150 | 121 | 97 | 101 |
| Cr | 0.011 | 0.09 | 0.013 | 0.011 | 0.007 | 0.008 | 0.002 | 0.001 | 0.012 | 0.011 |
| Ni/Co | 4.34 | 4.52 | 6.67 | 8.10 | 20.00 | 9.57 | 10.74 | 8.75 | 8.70 | 11.74 |
| U/Th | 0.19 | 0.17 | 0.16 | 0.15 | 0.25 | 0.25 | 0.21 | 0.18 | 0.21 | 0.19 |
| V/(V+Ni) | 0.84 | 0.87 | 0.88 | 0.87 | 0.84 | 0.87 | 0.84 | 0.85 | 0.83 | 0.79 |
| Co/Th | 4.08 | 3.82 | 2.14 | 2.1 | 1.25 | 2.56 | 2.25 | 1.85 | 2.30 | 2.09 |
| La/Th | 3.15 | 2.92 | 3.13 | 3.63 | 2.84 | 2.93 | 4.43 | 3.79 | 2.88 | 2.57 |
| Sr/Cu | 1.51 | 1.73 | 33.70 | 10.23 | 15.48 | 11.35 | 0.87 | 1.36 | 6.28 | 5.71 |
| Ga/Rb | 0.12 | 0.12 | 0.13 | 0.09 | 0.10 | 0.11 | 0.12 | 0.11 | 0.11 | 0.12 |
| Sample No. | EFBa | EFZr | EFRb | EFV | EFSr | EFPb | EFAs | EFNi | EFGa | EFTh | EFSc | EFCu | EFCs | EFU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.43 | 1.46 | 1.32 | 1.00 | 0.12 | 0.97 | 0.68 | 0.35 | 1.16 | 1.61 | 1.22 | 0.55 | 3.74 | 1.32 |
| 2 | 0.34 | 1.50 | 1.15 | 0.97 | 0.10 | 0.96 | 0.53 | 0.27 | 0.98 | 1.55 | 1.04 | 0.41 | 3.02 | 1.14 |
| 3 | 0.18 | 1.39 | 0.98 | 0.92 | 0.09 | 0.32 | 0.09 | 0.24 | 0.92 | 1.64 | 1.08 | 0.01 | 1.52 | 1.09 |
| 4 | 0.21 | 1.30 | 0.96 | 0.79 | 0.09 | 0.45 | 0.13 | 0.23 | 0.64 | 1.65 | 1.14 | 0.06 | 1.57 | 1.03 |
| 5 | 0.99 | 2.36 | 1.30 | 1.13 | 0.15 | 3.57 | 6.90 | 0.40 | 0.92 | 1.46 | 1.28 | 0.06 | 1.90 | 1.59 |
| 6 | 0.77 | 1.57 | 1.41 | 1.28 | 0.11 | 2.79 | 3.89 | 0.37 | 1.17 | 1.11 | 1.07 | 0.06 | 1.62 | 1.20 |
| 7 | 0.88 | 1.52 | 1.74 | 1.54 | 0.13 | 0.91 | 1.26 | 0.56 | 1.52 | 2.05 | 1.54 | 1.07 | 2.28 | 1.85 |
| 8 | 0.47 | 1.34 | 1.38 | 1.16 | 0.18 | 0.79 | 1.29 | 0.38 | 1.12 | 1.84 | 1.35 | 0.90 | 2.35 | 1.44 |
| 9 | 0.31 | 2.02 | 0.94 | 0.68 | 0.15 | 1.55 | 5.29 | 0.27 | 0.78 | 1.03 | 0.63 | 0.15 | 1.5 | 0.92 |
| 10 | 0.37 | 2.26 | 1.02 | 0.84 | 0.135 | 1.49 | 6.13 | 0.43 | 0.87 | 1.10 | 0.92 | 0.15 | 1.6 | 0.89 |
| Average | 0.50 | 1.67 | 1.22 | 1.03 | 0.13 | 1.38 | 2.62 | 0.35 | 1.01 | 1.50 | 1.13 | 0.34 | 2.1 | 1.2 |
| Elements | Sample-1 | Sample-2 | Sample-3 | Sample-4 | Sample-5 | Sample-6 | Sample-7 | Sample-8 | Sample-9 | Sample-10 |
|---|---|---|---|---|---|---|---|---|---|---|
| La | 57.60 | 56.10 | 74.40 | 77.30 | 35.80 | 33.70 | 81.90 | 66.70 | 38.90 | 31.40 |
| Ce | 113.50 | 101.20 | 142.20 | 121.90 | 72.70 | 89.10 | 141.10 | 111.20 | 80.60 | 99.30 |
| Pr | 12.24 | 17.40 | 16.10 | 9.40 | 7.63 | 13.20 | 15.30 | 19.10 | 10.60 | 12.50 |
| Nd | 44.50 | 49.50 | 57.70 | 39.30 | 28.40 | 56.10 | 64.20 | 63.90 | 31.70 | 29.80 |
| Sm | 8.37 | 7.60 | 10.14 | 11.40 | 5.66 | 6.69 | 10.97 | 9.71 | 6.23 | 5.78 |
| Eu | 1.60 | 1.20 | 1.90 | 1.70 | 1.23 | 2.20 | 2.07 | 1.98 | 1.35 | 1.21 |
| Gd | 7.27 | 6.30 | 8.21 | 7.74 | 5.24 | 6.10 | 8.65 | 4.78 | 5.81 | 5.01 |
| Tb | 1.08 | 1.01 | 1.20 | 1.10 | 0.80 | 0.70 | 1.22 | 1.14 | 0.91 | 1.30 |
| Dy | 6.19 | 7.21 | 6.97 | 5.87 | 4.56 | 5.21 | 6.73 | 4.90 | 5.23 | 6.01 |
| Ho | 1.25 | 1.21 | 1.41 | 1.43 | 0.95 | 0.99 | 1.24 | 1.23 | 0.99 | 0.98 |
| Er | 3.46 | 3.21 | 4.13 | 4.01 | 2.78 | 3.31 | 3.63 | 2.87 | 3.02 | 2.61 |
| Tm | 0.52 | 0.49 | 0.60 | 0.45 | 0.41 | 0.57 | 0.52 | 0.39 | 0.44 | 0.53 |
| Yb | 3.36 | 3.21 | 3.94 | 3.71 | 2.67 | 3.40 | 3.50 | 2.12 | 2.86 | 2.76 |
| Lu | 0.51 | 0.55 | 0.60 | 0.48 | 0.42 | 0.50 | 0.54 | 0.41 | 0.46 | 0.39 |
| ΣREE | 261.45 | 256.19 | 329.50 | 285.79 | 169.25 | 221.77 | 341.57 | 290.43 | 189.10 | 199.58 |
| Average | 26.15 | 25.62 | 32.95 | 28.58 | 16.93 | 22.18 | 34.16 | 29.04 | 18.91 | 19.96 |
| ΣLREE | 237.81 | 233.00 | 302.44 | 261.00 | 151.42 | 200.99 | 315.54 | 272.59 | 169.38 | 179.99 |
| ΣHREE | 23.64 | 23.19 | 27.06 | 24.79 | 17.83 | 20.78 | 26.03 | 17.84 | 19.72 | 19.59 |
| LREE/HREE | 10.06 | 10.05 | 11.18 | 10.53 | 8.49 | 9.67 | 12.12 | 15.28 | 8.59 | 9.19 |
| (La/La*)N | 1.13 | 0.53 | 1.08 | 2.86 | 1.18 | 0.94 | 1.88 | 0.72 | 0.64 | 0.35 |
| (Ce/Ce*)N | 0.95 | 1.14 | 0.96 | 0.68 | 0.95 | 1.25 | 0.89 | 1.10 | 1.08 | 1.26 |
| (Pr/Pr*)N | 0.98 | 1.39 | 1.01 | 0.77 | 0.95 | 1.03 | 0.91 | 1.27 | 1.19 | 1.29 |
| (Eu/Eu*)N | 0.94 | 1.46 | 1.05 | 0.58 | 0.84 | 1.35 | 0.94 | 1.30 | 1.05 | 1.12 |
| (Gd/Yb)N | 1.29 | 1.17 | 1.24 | 1.24 | 1.17 | 1.07 | 1.47 | 1.34 | 1.21 | 1.08 |
| (La/Yb)N | 1.26 | 1.29 | 1.39 | 1.54 | 0.99 | 0.73 | 1.72 | 2.32 | 1.00 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajid, Z.; Ismail, M.S.; Zakariah, M.N.A.; Tsegab, H.; Gámez Vintaned, J.A.; Hanif, T.; Ahmed, N. Impact of Paleosalinity, Paleoredox, Paleoproductivity/Preservation on the Organic Matter Enrichment in Black Shales from Triassic Turbidites of Semanggol Basin, Peninsular Malaysia. Minerals 2020, 10, 915. https://0-doi-org.brum.beds.ac.uk/10.3390/min10100915
Sajid Z, Ismail MS, Zakariah MNA, Tsegab H, Gámez Vintaned JA, Hanif T, Ahmed N. Impact of Paleosalinity, Paleoredox, Paleoproductivity/Preservation on the Organic Matter Enrichment in Black Shales from Triassic Turbidites of Semanggol Basin, Peninsular Malaysia. Minerals. 2020; 10(10):915. https://0-doi-org.brum.beds.ac.uk/10.3390/min10100915
Chicago/Turabian StyleSajid, Zulqarnain, Mohd S. Ismail, Muhammad Noor Amin Zakariah, Haylay Tsegab, José Antonio Gámez Vintaned, Tanzila Hanif, and Nisar Ahmed. 2020. "Impact of Paleosalinity, Paleoredox, Paleoproductivity/Preservation on the Organic Matter Enrichment in Black Shales from Triassic Turbidites of Semanggol Basin, Peninsular Malaysia" Minerals 10, no. 10: 915. https://0-doi-org.brum.beds.ac.uk/10.3390/min10100915