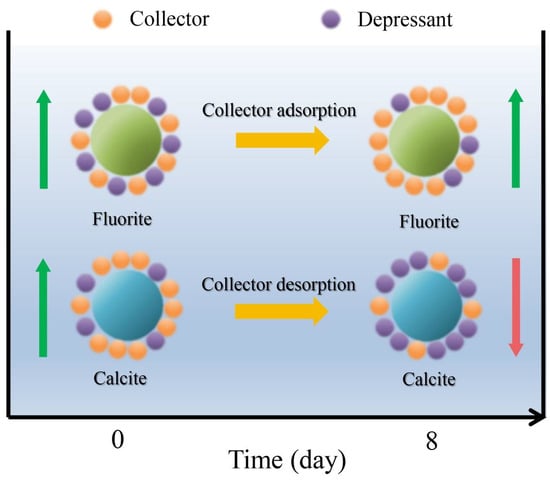
The Contribution of Long-Terms Static Interactions Between Minerals and Flotation Reagents for the Separation of Fluorite and Calcite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Micro-Flotation Tests of Single Mineral
2.3. Collector Adsorption Measurements
2.4. Zeta Potential Measurements
2.5. Contact Angle Measurements
2.6. FTIR and XPS Analyses
2.7. Flotation Tests of Tailings
3. Results and Discussion
3.1. The Flotation Separation of Fluorite Form Calcite Improved by the Long-Term Static Interaction
3.2. Effect of Static Interaction Time on the Amount of Collector Adsorbed on Fluorite
3.3. The Separation of Fluorite and Calcite Facilitated by the Adsorbed Water Glass on Calcite
3.4. The Hydrophilicity of Calcite Surface Enhanced after 8 Days of Static Interaction
3.5. Roles of Si–O and –OH of Water Glass and C=O of OA in the Separation between Fluorite and Calcite
3.6. Effect of Static Interaction Time on the Amount of OA in Collector Adsorbed on Calcite
3.7. Effect of Long-Term Interaction on the Flotation Separation between Fluorite and Calcite in Tailings
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, W.; Gao, Z.; Sun, W.; Gao, J.; Hu, Y. A Density Functional Theory Study on the Effect of Lattice Impurities on the Electronic Structures and Reactivity of Fluorite. Minerals 2017, 7, 160. [Google Scholar] [CrossRef]
- Liu, S.; Lu, X.; Wei, S. Study on Flotation Process of Low Quality Fluorite ore. Adv. Mater. Res. 2013, 753–755, 36–39. [Google Scholar] [CrossRef]
- Li, Y.J.; Sun, F.Y.; Zhou, Y.; Zeng, L. The Improvement Effect of Dispersant in Fluorite Flotation: Determination by the Analysis of XRD and FESEM-EDX. J. Spectrosc. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Q.; Guo, Y.; Xiao, H.; Jiang, M. Research of the Low Grade Fluorite Flotation Orthogonal Test. Adv. Mater. Res. 2013, 734–737, 1068–1072. [Google Scholar] [CrossRef]
- Zheng, R.; Ren, Z.; Gao, H.; Chen, Z.; Qian, Y.; Li, Y. Effects of crystal chemistry on sodium oleate adsorption on fluorite surface investigated by molecular dynamics simulation. Miner. Eng. 2018, 124, 77–85. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, Y.; Zhu, Y.; Hu, Y.; Sun, W. Selective Flotation of Calcite from Fluorite: A Novel Reagent Schedule. Minerals 2016, 6, 114. [Google Scholar] [CrossRef]
- Yarar, B.; Richter, R.B. Flotation. In Ullmann’s Encyclopedia of Industrial Chemistry, 7th ed.; Elvers, B., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar] [CrossRef]
- Fa, K.; Jiang, T.; Nalaskowski, J.; Miller, J.D. Interaction Forces between a Calcium Dioleate Sphere and Calcite/Fluorite Surfaces and Their Significance in Flotation. Langmuir 2003, 19, 10523–10530. [Google Scholar] [CrossRef]
- Zhang, C.; Wei, S.; Hu, Y.; Tang, H.; Gao, J.; Yin, Z.; Guan, Q. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.S.; Somasundaran, P. Flotation of Salt-Type Minerals, 8th ed.; Fuerstenau, M.C., Ed.; American Institute of Mining, Metallurgical and Petroleum Engineers: New York, NY, USA, 1976; Flotation--A.M. Gaudin Memorial Volume; pp. 197–272. [Google Scholar]
- Finkelstein, N.P. Review of interactions in flotation of sparingly soluble calcium minerals with anionic collectors. Trans. Inst. Min. Metall. 1989, 98, 157–177. [Google Scholar]
- Somasundaran, P.; Healy, T.W.; Fuerstenau, D.W. Surfactant adsorption at the solid-liquid interface-dependence of mechanism on chain length. J. Phys. Chem. 1964, 68, 3562–3566. [Google Scholar] [CrossRef]
- Chennakesavulu, K.; Raju, G.B.; Prabhakar, S.; Nair, C.M.; Murthy, K.V.G.K. Adsorption of oleate on fluorite surface as revealed by atomic force microscopy. Int. J. Miner. Process. 2009, 90, 101–104. [Google Scholar] [CrossRef]
- Zheng, R.; Ren, Z.; Gao, H.; Qian, Y. Flotation Behavior of Different Colored Fluorites Using Sodium Oleate as a Collector. Minerals 2017, 7, 159. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Laskowski, J.S. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction. Int. J. Miner. Process. 2000, 60, 229–245. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, F.; Gao, H.; Chen, Z.; Peng, Y.; Liu, L. Selective Separation of Fluorite, Barite and Calcite with Valonea Extract and Sodium Fluosilicate as Depressants. Minerals 2017, 7, 24. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Bunge, R.C. The complex behavior of fatty acids in fluorite flotation. XXXIII Miner. Process. Congress 2006, 1, 510–515. [Google Scholar]
- Helbig, C.; Baldauf, H.; Mahnke, J.; Stöckelhuber, K.W.; Schulze, H.J. Investigation of Langmuir monofilms and flotation experiments with anionic/cationic collector mixtures. Int. J. Miner. Process 1998, 53, 135–144. [Google Scholar] [CrossRef]
- Helbig, C.; Baldauf, H.; Lange, T.; Newmann, R.; Pollex, R.; Weber, E. New binary collectors with increased flotation efficiency. Tenside Surfact. Deterg. 1999, 36, 58–62. [Google Scholar]
- Zhang, G.; Gao, Y.; Chen, W.; Liu, D. The Role of Water Glass in the Flotation Separation of Fine Fluorite from Fine Quartz. Minerals 2017, 7, 157. [Google Scholar] [CrossRef]
- Pugh, R.; Stenius, P. Solution chemistry studies and flotation behaviour of apatite, calcite and fluorite minerals with sodium oleate collector. Int. J. Miner. Process 1985, 15, 193–218. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Jin, H.; Hu, X.; Qi, M. A study on Molecular Mechanics Analysis Flotation Collector in the process management Fluorite Tailing Mining. Appl. Mech. Mater. 2014, 577, 1223–1227. [Google Scholar] [CrossRef]
- Alghunaim, A.; Kirdponpattara, S.; Newby, B.-M.Z. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Dong, X.; Zong, Q.; He, J. Anisotropic surface properties and wettability of disperse dye single crystal. Dyes Pigments 2013, 9, 636–641. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, L.; Sun, W.; Hu, Y.; Han, H. Surface characteristics and wettability enhancement of respirable sintering dust by nonionic surfactant. Colloids Surf. A 2016, 509, 323–333. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Shergold, H.L. The mechanism of fatty acid adsorption in the presence of fluorite, calcite and barite. Int. J. Miner. Process 1985, 14, 161–176. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Jiang, H.; Li, Y.; Zhou, S. Effects of grinding media on flotation performance of calcite. Miner. Eng. 2019, 132, 92–94. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Herrera-Urbina, R. The surface chemistry of bastnaesite, barite and calcite in aqueous carbonate solutions. Colloids Surf. 1992, 68, 95–102. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Shergold, H.L. Influence of sodium silicate addition on the adsorption of oleic acid by fluorite, calcite and barite. Int. J. Miner. Process 1985, 14, 177–193. [Google Scholar] [CrossRef]
- Gao, J.; Hu, Y.; Sun, W.; Liu, R.; Gao, Z.; Han, H.; Lyu, F.; Jiang, W. Enhanced separation of fluorite from calcite in acidic condition. Miner. Eng. 2019, 133, 103–105. [Google Scholar] [CrossRef]
- Dadson, J.; Pandam, S.; Asiedu, N. Modeling the characteristics and quantification of adulterants in gasoline using FTIR spectroscopy and chemometric calibrations. Cogent. Chem. 2018, 4, 1–22. [Google Scholar] [CrossRef]
- He, T.; Li, H.; Jin, J.; Peng, Y.; Wang, Y.; Wan, H. Improving fine molybdenite flotation using a combination of aliphatic hydrocarbon oil and polycyclic aromatic hydrocarbon. Results Phys. 2019, 12, 1050–1055. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Thys, R.C.S.; Brandelli, A. Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. Int. J. Food Microbiol. 2019, 293, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gholoobi, A.; Abnous, K.; Ramezani, M.; Shandiz, F.H.; Darroudi, M.; Ghayour-Mobarhan, M.; Meshkat, Z. Synthesis of γ-Fe2O3 Nanoparticles Capped with Oleic Acid and their Magnetic Characterization. Iran. J. Sci. Technol. A 2017, 42, 1889–1893. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.; Ma, S.; Liu, C.; Ding, J.; Wang, X. Novel two-step process for synthesising β-SiC whiskers from coal fly ash and water glass. Ceram. Int. 2018, 44, 10585–10595. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q. Investigations on flotation separation of scheelite from calcite by using a novel depressant: Sodium phytate. Miner. Eng. 2018, 126, 116–122. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, W.; Chen, C.; Chai, L.; Jiao, F.; Jia, W. Flotation separation of fluorite from calcite using polyaspartate as depressant. Miner. Eng. 2018, 120, 80–86. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.; Han, H.; Sun, W.; Wang, R.; Wang, J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]











| Composition | SiO2 | TiO2 | Al2O3 | FeOx | MnO | CaO | MgO | K2O | Na2O | P2O5 | CaF2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 72.22 | 0.10 | 9.25 | 2.62 | 0.03 | 2.45 | 0.20 | 2.30 | 2.20 | 0.08 | 8.55 |
| Mineral Sample | m (g) | V (L) | C0 (mg/L) | 0 Days | 8 Days | ||
|---|---|---|---|---|---|---|---|
| C1 (mg/L) | Γ (mg/g) | C2 (mg/L) | Γ (mg/g) | ||||
| Fluorite | 2 | 0.035 | 32.18 | 9.577 | 0.396 | 8.143 | 0.421 |
| Calcite | 2 | 0.035 | 32.18 | 6.799 | 0.444 | 9.110 | 0.404 |
| Mineral Samples | Experimental Condition | ||
|---|---|---|---|
| No Reagent | Reagents for 0 Days | Reagents for 8 Days | |
| Fluorite | 44.3° ± 3.5° | 134.3° ± 4.2° | 109.7° ± 3.3° |
| Calcite | 45.9° ± 3.6° | 105.0° ± 3.0° | 52.5° ± 5.0° |
| Concentrates | 0 Day | 8 Days | ||
|---|---|---|---|---|
| Grade (%) | Recovery (%) | Grade (%) | Recovery (%) | |
| Fluorite | 87.18 | 40.99 | 93.00 | 46.01 |
| Calcite | 48.28 | 44.36 | 44.39 | 42.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zeng, Q.; Hu, L.; Hu, Y.; Zhong, H.; He, Z. The Contribution of Long-Terms Static Interactions Between Minerals and Flotation Reagents for the Separation of Fluorite and Calcite. Minerals 2019, 9, 699. https://0-doi-org.brum.beds.ac.uk/10.3390/min9110699
Huang L, Zeng Q, Hu L, Hu Y, Zhong H, He Z. The Contribution of Long-Terms Static Interactions Between Minerals and Flotation Reagents for the Separation of Fluorite and Calcite. Minerals. 2019; 9(11):699. https://0-doi-org.brum.beds.ac.uk/10.3390/min9110699
Chicago/Turabian StyleHuang, Leiming, Qiang Zeng, Liang Hu, Yuehua Hu, Hui Zhong, and Zhiguo He. 2019. "The Contribution of Long-Terms Static Interactions Between Minerals and Flotation Reagents for the Separation of Fluorite and Calcite" Minerals 9, no. 11: 699. https://0-doi-org.brum.beds.ac.uk/10.3390/min9110699