Synthesis and Biological Evaluation of Novel Benzothiazole Derivatives as Potential Anticonvulsant Agents
Abstract
:1. Introduction

2. Results and Discussion
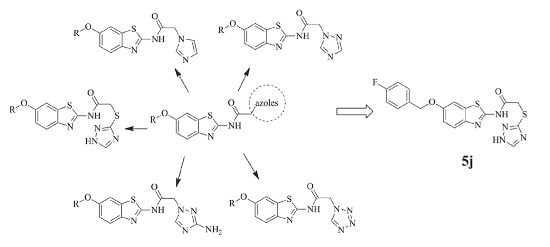
2.1. Chemistry

2.2. Pharmacology and Structure-Activity Relationship

| Comp. | R | MES (100 mg/kg) a | Toxicity (100 mg/kg) | ||
|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | ||
| 5a | -CH3 | 1/3 b | 0/3 | 0/3 | 0/3 |
| 5b | n-C3H7 | 3/3 | 0/3 | 0/3 | 0/3 |
| 5c | n-C4H9 | 3/3 | 1/3 | 0/3 | 0/3 |
| 5d | n-C5H11 | 0/3 | 0/3 | 0/3 | 0/3 |
| 5e | n-C6H13 | 1/3 | 0/3 | 0/3 | 0/3 |
| 5f | n-C7H15 | 1/3 | 0/3 | 0/3 | 0/3 |
| 5g | -CH2C6H5 | 3/3 | 0/3 | 0/3 | 0/3 |
| 5h | -CH2C6H4 (o-F) | 2/3 | 0/3 | 0/3 | 0/3 |
| 5i | -CH2C6H4 (m-F) | 3/3 | 1/3 | 0/3 | 0/3 |
| 5j | -CH2C6H4 (p-F) | 3/3 | 1/3 | 0/3 | 0/3 |
| 5k | -CH2C6H4 (o-Cl) | 0/3 | 0/3 | 0/3 | 0/3 |
| 5l | -CH2C6H4 (m-Cl) | 0/3 | 0/3 | 0/3 | 0/3 |
| 5m | -CH2C6H4 (m-CF3) | 0/3 | 0/3 | 0/3 | 0/3 |
| Comp. | R | MES (30 mg/kg) a | |
|---|---|---|---|
| 0.5 h | 4 h | ||
| 5b | n-C3H7 | 0/3 | 0/3 |
| 5c | n-C4H9 | 0/3 | 0/3 |
| 5g | -CH2C6H5 | 0/3 | 0/3 |
| 5h | -CH2C6H4 (o-F) | 0/3 | 0/3 |
| 5i | -CH2C6H4 (m-F) | 1/3 | 0/3 |
| 5j | -CH2C6H4 (p-F) | 1/3 | 0/3 |
| Comp. | R | MES (100 mg/kg) | Toxicity (100 mg/kg) | ||
|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | ||
| 6a | -CH2C6H4 (m-F) | 0/3 | 0/3 | 0/3 | 0/3 |
| 6b | -CH2C6H4 (p-F) | 0/3 | 0/3 | 0/3 | 0/3 |
| 7a | -CH2C6H4 (m-F) | 0/3 | 0/3 | 0/3 | 0/3 |
| 7b | -CH2C6H4 (p-F) | 0/3 | 0/3 | 0/3 | 0/3 |
| 8a | -CH2C6H4 (m-F) | 0/3 | 0/3 | 0/3 | 0/3 |
| 8b | -CH2C6H4 (p-F) | 0/3 | 0/3 | 0/3 | 0/3 |
| 9a | -CH2C6H4 (m-F) | 1/3 | 1/3 | 0/3 | 0/3 |
| 9b | -CH2C6H4 (p-F) | 0/3 | 0/3 | 0/3 | 0/3 |
| Comp. | ED50 a MES (mg/kg) | ED50 scPTZ b (mg/kg) | TD50 c (mg/kg) | PI d | |
|---|---|---|---|---|---|
| MES | scPTZ | ||||
| 5i | 50.8 (37.0–69.8) e | 76.0 (65.9–87.7) | 353.5 (309.3–404.1) | 6.96 | 4.65 |
| 5j | 54.8 (36.7–81.8) | 52.8 (45.8–60.9) | 491.0 (429.5–561.2) | 8.96 | 9.30 |
| CBZ | 11.8 (8.5–16.4) | >100 | 76.1 (55.8–103.7) | 6.45 | <0.76 |
| VPA | 216.9 (207.5–226.3) | 239.4 (209.2–274.1) | 372.9 (356.0–389.8) | 1.72 | 1.56 |
3. Experimental Procedures
3.1. General Information
3.2. Chemistry
3.2.1. Synthesis of 6-Hydroxy-2-aminobenzothiazole (2)
3.2.2. General Procedure for the Synthesis of 6-Alkoxy-2-aminobenzothiazoles (3a–m)
3.2.3. General Procedure for the Synthesis of 2-Chloro-N-(6-alkoxybenzo[d]thiazol-2-yl)acetamide (4a–m)
3.2.4. General Procedure for the Synthesis of 2-((1H-1,2,4-Triazol-3-yl)thio)-N-(6-alkoxybenzo[d]thiazol-2-yl)acetamide (5a–m)
3.2.5. General Procedure for the Synthesis of N-(6-Alkoxybenzo[d]thiazol-2-yl)-2-(1H-imidazol-1-yl)acetamide (6a–b)
3.2.6. General Procedure for the Synthesis of N-(6-Alkoxybenzo[d]thiazol-2-yl)-2-(1H-1,2,4-triazol-1-yl)acetamide (7a–b)
3.2.7. General Procedure for the Synthesis of N-(6-Alkoxybenzo[d]thiazol-2-yl)-2-(1H-tetrazol-1-yl)acetamide (8a–b)
3.2.8. General Procedure for the Synthesis of 2-(3-Amino-1H-1,2,4-triazol-1-yl)-N-(6-alkoxybenzo[d]thiazol-2-yl)acetamide (9a–b)
3.3. Pharmacology
3.3.1. MES Screening Test
3.3.2. ScPTZ Seizures Screening Test
3.3.3. Neurotoxicity Screening Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, B.; Raedt, R.; Sprengers, M.; Dauwe, I.; Gadeyne, S.; Carrette, E.; Delbeke, J.; Wadman, W.J.; Boon, P.; Vonck, K. The systemic kainic acid rat model of temporal lobe epilepsy: Long-term EEG monitoring. Brain Res. 2015, 1627, 1–11. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. A voice for people with epilepsy. Lancet 2015, 385, 482. [Google Scholar]
- Deng, X.Q.; Song, M.X.; Zheng, Y.; Quan, Z.S. Design, synthesis and evaluation of the antidepressant and anticonvulsant activities of triazole-containing quinolinones. Eur. J. Med. Chem. 2014, 73, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sarigol, D.; Uzgoren-Baran, A.; Tel, B.C.; Somuncuoglu, E.I.; Kazkayasi, I.; Ozadali-Sari, K.; Unsal-Tan, O.; Okay, G.; Ertan, M.; Tozkoparan, B. Novel thiazolo[3,2−b]-1,2,4-triazoles derived from naproxen with analgesic/anti-inflammatory properties: Synthesis; biological evaluation and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Ahmad, Z.; Sharma, S.; Khuller, G.K. Nano-encapsulation of azole antifungals: Potential applications to improve oral drug delivery. Int. J. Pharm. 2005, 301, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Barradas, J.S.; Errea, M.I.; D’Accorso, N.B.; Sepúlveda, C.S.; Talarico, L.B.; Damonte, E.B. Synthesis and antiviral activity of azoles obtained from carbohydrates. Carbohydr. Res. 2008, 343, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Chandra, S.; Saraswat, B.S.; Yadav, D. Design, spectral characterization; thermal; DFT studies and anticancer cell line activities of Co(II); Ni(II) and Cu(II) complexes of Schiff bases derived from 4-amino-5-(pyridin-4-yl) 4H-1;2;4-triazole-3-thiol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 145, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, H.; Abnosi, M.H.; Hosseinzadeh, A. Synthesis, biological and computational study of new Schiff base hydrazones bearing 3-(4-pyridine)-5-mercapto-1,2,4-triazole moiety. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 71, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.M.; Vella, P.; Islam, N.U.; Ollis, D.L.; Schenk, G.; McGeary, R.P. 3-Mercapto-1,2,4-triazoles and N-acylated thiosemicarbazides as metallo-β-lactamase inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 380–386. [Google Scholar]
- Agarwal, S.; Agarwal, D.K.; Gautam, N.; Agarwal, K.; Gautam, D.C. Synthesis and in vitro antimicrobial evaluation of benzothiazole incorporated thiazolidin-4-ones derivatives. J. Korean Chem. Soc. 2014, 58, 33–38. [Google Scholar] [CrossRef]
- Singh, M.K.; Tilak, R.; Nath, G.; Awasthi, S.K.; Agarwal, A. Design, synthesis and antimicrobial activity of novel benzothiazole analogs. Eur. J. Med. Chem. 2013, 63, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.L.; Citossi, F.; Singh, R.; Kaur, B.; Gaskell, M.; Farmer, P.B.; Monks, A.; Hose, C.; Stevens, M.F.; Leong, C.O.; et al. Antitumour benzothiazoles. Part 32: DNA adducts and double strand breaks correlate with activity; synthesis of 5F203 hydrogels for local delivery. Bioorg. Med. Chem. 2015, 23, 6891–6899. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Bao, G.; Wang, L.; Li, W.; Xu, B.; Du, B.; Lv, J.; Zhai, X.; Gong, P. Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur. J. Med. Chem. 2015, 96, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Ugale, V.G.; Patel, H.M.; Wadodkar, S.G.; Bari, S.B.; Shirkhedkar, A.A.; Surana, S.J. Quinazolino-benzothiazoles: Fused pharmacophores as anticonvulsant agents. Eur. J. Med. Chem. 2012, 53, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Bhat, M.A.; Haque, S.E. Synthesis of benzothiazole semicarbazones as novel anticonvulsants—The role of hydrophobic domain. Bioorg. Med. Chem. Lett. 2007, 17, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Khan, S.A.; Amir, M. Design; synthesis and evaluation of N-(substituted benzothiazol-2-yl)amides as anticonvulsant and neuroprotective. Eur. J. Med. Chem. 2012, 58, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, L.; Kumar, P.; Singh, R.; Stables, J.P. Design, synthesis and anticonvulsant evaluation of novel N-(4-substituted phenyl)-2-[4-(substituted) benzylidene]-hydrazinecarbothio amides. Eur. J. Med. Chem. 2012, 47, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.C.; Deng, X.Q.; Wang, S.B.; Quan, Z.S. Synthesis and anticonvulsant activity evaluation of 7-alkoxy[1,2,4]triazolo[3,4-b]benzothiazol-3(2H)-ones. Arch. Pharm. Chem. Life Sci. 2014, 347, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, H.; Ibrahim, H.M. 4-Thiazolidinones in heterocyclic synthesis: Synthesis of novel enaminones, azolopyrimidines and 2-arylimino-5-arylidene-4-thiazolidinones. Molecules 2012, 17, 6362–6385. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, A.H.; Gouda, A.M.; Omar, H.A.; Tolba, M.F. Design; synthesis and biological evaluation of novel diphenylthiazole-based cyclooxygenase inhibitors as potential anticancer agents. Bioorg. Chem. 2014, 57, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Yang, K.W.; Zhou, Y.J.; La Curan, A.E.; Oelschlaeger, P.; Crowder, M.W. Diaryl-substituted azolylthioacetamides: Inhibitor discovery of New Delhi metallo-β-lactamase-1 (NDM-1). ChemMedChem 2014, 9, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.F.; Xu, L.; Lu, A.H. Synthesis and bioactivity of novel triazolo [1,5−a] pyrimidine derivatives. Heteroatom Chem. 2001, 12, 491–496. [Google Scholar] [CrossRef]
- Deb, P.K.; Kaur, R.; Chandrasekaran, B.; Bala, M.; Gill, D.; Kaki, V.R.; Akkinepalli, R.R.; Mailavaram, R. Synthesis, anti-inflammatory evaluation, and docking studies of some new thiazole derivatives. Med. Chem. Res. 2014, 23, 2780–2792. [Google Scholar] [CrossRef]
- Papadopoulou, M.V.; Bloomer, W.D.; Rosenzweig, H.S.; Chatelain, E.; Kaiser, M.; Wilkinson, S.R.; McKenzie, C.; Ioset, J.R. Novel 3-nitro-1H-1,2,4-triazole- based amides and sulfonamides as potential antitrypanosomal agents. J. Med. Chem. 2012, 55, 5554–5565. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A. Molecular targets versus models for new antiepileptic drug discovery. Epilepsy Res. 2006, 68, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Castel-Branco, M.M.; Alves, G.L.; Figueiredo, I.V.; Falcão, A.C.; Caramona, M.M. The maximal electroshock seizure (MES) model in the preclinical assessment of potential new antiepileptic drugs. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure 2011, 20, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Krall, R.L.; Penry, J.K.; White, B.G.; Kupferberg, H.J.; Swinyard, E.A. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Cereghino, J.J.; Gladding, G.D.; Hessie, B.J.; Kupferberg, H.J.; Scoville, B.; White, B.G. Antiepileptic Drug Development Program. Cleve. Clin. Q. 1984, 51, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all the target compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-C.; Zhang, H.-J.; Jin, C.-M.; Quan, Z.-S. Synthesis and Biological Evaluation of Novel Benzothiazole Derivatives as Potential Anticonvulsant Agents. Molecules 2016, 21, 164. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21030164
Liu D-C, Zhang H-J, Jin C-M, Quan Z-S. Synthesis and Biological Evaluation of Novel Benzothiazole Derivatives as Potential Anticonvulsant Agents. Molecules. 2016; 21(3):164. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21030164
Chicago/Turabian StyleLiu, Da-Chuan, Hong-Jian Zhang, Chun-Mei Jin, and Zhe-Shan Quan. 2016. "Synthesis and Biological Evaluation of Novel Benzothiazole Derivatives as Potential Anticonvulsant Agents" Molecules 21, no. 3: 164. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21030164