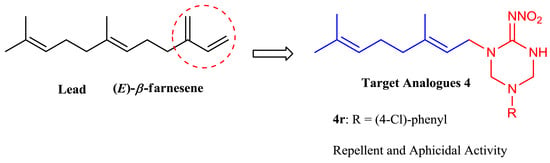
Novel (E)-β-Farnesene Analogues Containing 2-Nitroiminohexahydro-1,3,5-triazine: Synthesis and Biological Activity Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Activity
2.2.1. Repellent Activity
2.2.2. Aphicidal Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis of EβF Analogues 4a–4t
3.2.1. General Procedure for the Preparation of Intermediate 2
3.2.2. General Procedure for the Preparation of Intermediate 3a–3t
3.2.3. General Procedure of EβF Analogues 4a–4t
3.3. X-ray Diffraction
3.4. Stability Test
3.5. Biological Activity Test
3.5.1. Repellent Assays
3.5.2. Aphicidal Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dedryver, C.A.; Le, R.A.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. Comptes Rendus Biol. 2010, 33, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Bonnemain, J.-L. Aphids as biological models and agricultural pests. Comptes Rendus Biol. 2010, 333, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Allemann, R.K.; Birkett, M.A. The semiochemistry of aphids. Nat. Prod. Rep. 2013, 30, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.R.; Braman, S.K.; Williams-Woodward, J.; Knox, G. Optimizing plant health and pest management of Lagerstroemia spp. in commercial production and landscape situations in the Southeastern United States: A review. J. Environ. Hortic. 2012, 30, 161–172. [Google Scholar]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Casteel, C.L.; Yang, C.L.; Nanduri, A.C.; De Jong, H.N.; Whitham, S.A.; Jander, G. The NIa-Pro protein of Turnip mosaic virus improves growth and reproduction of the aphid vector, Myzus persicae (green peach aphid). Plant J. 2014, 77, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Linz, L.B.; Liu, S.J.; Chougule, N.P.; Bonning, B.C. In vitro evidence supports membrane alanyl aminopeptidase N as a receptor for a plant virus in the pea aphid vector. J. Virol. 2015, 89, 11203–11212. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Huang, W.Y.; Ling, Y.; Kan, W.; Fang, Y.L.; Zhang, Z.N. Design, synthesis and biological activity of EBF analogues. Chem. J. Chin. Univ. 2004, 25, 1657–1661. [Google Scholar]
- Zhang, Z.N.; Liu, X.; Mei, X.Q.; Meng, X.Y.; Pan, Y.C.; Zhao, J.X.; Li, W.X. Field performance of controlling aphids by using the mixture of aphid alarm pheromone and insecticides. Anim. Bull. 1993, 10, 1–5. [Google Scholar]
- Yan, F.M.; Chen, J.L.; Tang, Q.B. Advances and perspectives in insect chemical ecology. Plant Prot. 2013, 39, 9–15. [Google Scholar]
- Dahl, M.L. On an alarm substance in aphids. Dtsch. Ent. Z. 1971, 18, 121–127. [Google Scholar] [CrossRef]
- Kislow, C.J.; Edwards, L.J. Repellent odour in aphids. Nature 1972, 235, 108–109. [Google Scholar] [CrossRef]
- Nault, L.R.; Edwards, L.J.; Styer, W.E. Aphid alarm pheromones secretion and reception. Environ. Entomol. 1973, 2, 101–105. [Google Scholar] [CrossRef]
- Vandermoten, S.; Mescher, M.C.; Francis, F.; Haubruge, E.; Verheggen, F.J. Aphid alarm pheromone: An overview of current knowledge on biosynthesis and functions. Insect Biochem. Mol. Biol. 2012, 42, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Van, O.A.M.; Gut, J.; Harrewijn, P.; Piron, P.G.M. Role of farnesene isomers and other terpenoids in the development of different morphs and forms of the aphids Aphis-fabae and Myzus-persicae. Acta Phytopathol. Entomol. Hung. 1990, 25, 331–342. [Google Scholar]
- Cui, L.L.; Dong, J.; Francis, F.; Liu, Y.J.; Heuskin, S.; Lognay, G.; Chen, J.L.; Bragard, C.; Tooker, J.F.; Liu, Y. E-beta-farnesene synergizes the influence of an insecticide to improve control of cabbage aphids in China. Crop Prot. 2012, 35, 91–96. [Google Scholar] [CrossRef]
- Sun, C.W.; Wang, H.F.; Zhu, J.; Yang, D.R.; Jin, J.; Xing, J.H. Synthesis, insecticidal activities, and molecular docking studies of 1,5-disubstituted-1,3,5-hexahydrotriazine-2-(N-nitro)imines. J. Heterocycl. Chem. 2011, 48, 829–835. [Google Scholar] [CrossRef]
- Wu, K.; Kariya, A.; Katsuyama, N.; Tsuji, A.; Takasuka, K.; Segami, S.; Nanjo, K.; Sato, J. Hexahydrotriazine Compounds and Insecticides. U.S. Patent 6187773 B1, 13 February 2001. [Google Scholar]
- Maienfisch, P.; Kristiansen, O.; Gsell, L. Preparation of 2-(Nitroimino)-1,3,5-triazacyclohexane Pesticides. Patent EP 483055 A1, 29 April 1992. [Google Scholar]
- Shiokawa, K.; Tsuboi, S.; Moriya, K.; Hattori, Y.; Honda, I.; Shibuya, K. Insecticidal Heterocylic Compounds. U.S. Patent 6344453 B1, 5 February 2002. [Google Scholar]
- Cottrell, I.W.; Lytwyn, M.W.; Monro, C.M.; Ahn, A.; Joseph, P.R. High Concentration Topical Insecticide. U.S. Patent 6588374 B1, 8 July 2003. [Google Scholar]
- Sun, C.W.; Zhu, J.; Wang, H.F.; Jin, J.; Xing, J.H.; Yang, D.R. Chiral 1,5-disubstituted 1,3,5-hexahydrotriazine-2-N-nitroimine analogues as novel potent neonicotinoids: Synthesis, insecticidal evaluation and molecular docking studies. Eur. J. Med. Chem. 2011, 46, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Maienfisch, P.; Huerlimann, H.; Rindlisbacher, A.; Gsell, L.; Dettwiler, H.; Haettenschwiler, J.; Sieger, E.; Walti, M. The discovery of thiamethoxam: A second-generation neonicotinoid. Pest Manag. Sci. 2001, 57, 165–176. [Google Scholar] [CrossRef]
- Tsuboi, S.; Moriie, K.; Shibuya, K.; Sone, S.; Shiroshita, M. Preparation of Hexahydro-1,3,5-triazines as Pesticides. Patent JP 04243876 A, 31 August 1992. [Google Scholar]
- Shiokawa, K.; Tsuboi, S.; Moriya, K.; Hattori, Y.; Honda, I.; Shibuya, K. Heterocyclic Compounds. Patent EP 386565 A1, 9 December 1990. [Google Scholar]
- Griffiths, D.C.; Pickett, J.A. A potential application of aphid alarm pheromones. Entomol. Exp. Appl. 1980, 27, 199–201. [Google Scholar] [CrossRef]
- Chen, M.H.; Zhao, C.G.; Shen, J.G.; Zhou, Z.Y. Progresses in the synthesis and biological activity of triazine derivatives. J. Tongren Univ. 2013, 15, 137–143. [Google Scholar]
- Zhang, C.L.; Qu, Y.Y.; Wu, X.Q.; Song, D.L.; Ling, Y.; Yang, X.L. Eco-friendly insecticide discovery via peptidomimetics: Design, synthesis, and aphicidal activity of novel insect kinin analogues. J. Agric. Food Chem. 2015, 63, 4527–4532. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.N.; Ling, Y.; Rui, C.H.; Yang, X.L.; Fan, X.L.; Chen, F.H. Synthesis of E-β-Farnesene analogues containing five-membered azaheterocycles and their biological activity. Chin. J. Org. Chem. 2008, 28, 617–621. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr A. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Hori, M. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 1998, 24, 1425–1432. [Google Scholar] [CrossRef]
- Hori, M. Onion aphid (Neotoxoptera formosana) attractants, in the headspace of Allium fistulosum and A. tuberosum leaves. J. Appl. Entomol. 2007, 131, 8–12. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing of effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 4a–4t are available from the authors.




| Compound | 4r |
|---|---|
| Empirical formula | C10H13N5O3 |
| Formula weight | 251.25 |
| Temperature/K | 180.15 |
| Crystal system | monoclinic |
| Space group | P21/c |
| a/Å | 15.4268(13) |
| b/Å | 5.3261(4) |
| c/Å | 14.6589(11) |
| α/° | 90 |
| β/° | 110.045(9) |
| γ/° | 90 |
| Volume/Å3 | 1131.48(16) |
| Z | 4 |
| ρ calc mg/mm3 | 1.475 |
| m/mm−1 | 0.113 |
| F(000) | 528.0 |
| Crystal size/mm3 | 0.2 × 0.1 × 0.1 |
| 2Θ range for data collection | 7.372° to 50.036° |
| Index ranges | −15 ≤ h ≤ 16, −6 ≤ k ≤ 3, −14 ≤ l ≤ 17 |
| Reflections collected | 2423 |
| Independent reflections | 1780[R(int) = 0.0173] |
| Data/restraints/parameters | 1780/0/164 |
| Goodness-of-fit on F2 | 1.086 |
| Final R indexes (I ≥ 2σ (I)) | R1 = 0.0371, wR2 = 0.0914 |
| Final R indexes [all data] | R1 = 0.0480, wR2 = 0.0996 |
| Largest diff. peak/hole/e Å−3 | 0.16/−0.22 |
| CCDC No. | 1437627 |
| Compd. | R | RP (%) a | Compd. | R | RP (%) a |
|---|---|---|---|---|---|
| 4a | CH3 | 52.28 ± 2.40 a | 4l | (4-Cl)-benzyl | 67.56 ± 2.77 f,g |
| 4b | CH2CH3 | 56.74 ± 1.37 b | 4m | (2-CH3)-phenyl | 75.62 ± 2.16 h |
| 4c | (CH2)2CH3 | 60.89 ± 1.41 c | 4n | (3-CH3)-phenyl | 67.28 ± 1.66 f |
| 4d | (CH2)3CH3 | 64.03 ± 1.40 e | 4o | (4-CH3)-phenyl | 69.47 ± 1.91 g |
| 4e | (CH2)4CH3 | 55.96 ± 1.54 b | 4p | (4-CH2CH3)-phenyl | 67.79 ± 1.81 f,g |
| 4f | CH(CH3)2 | 61.34 ± 1.42 c | 4q | (4-F)-phenyl | 74.11 ± 2.74 h |
| 4g | CHCH2(CH3)2 | 63.89 ± 1.42 d,e | 4r | (4-Cl)-phenyl | 78.43 ± 2.00 i |
| 4h | C(CH3)3 | 62.89 ± 1.48 c–−e | 4s | (4-OCH3)-phenyl | 67.89 ± 2.24 f,g |
| 4i | cyclohexyl | 67.16 ± 2.09 f | 4t | (2,4-(CH3)2)-phenyl | 80.07 ± 2.20 i |
| 4j | phenyl | 62.57 ± 1.71 c–−e | EβF | - | 96.47 ± 2.31 j |
| 4k | benzyl | 61.93 ± 1.69 c,d |
| Compd. | R | Mortality (%) a (48 h) | Compd. | R | Mortality (%) a (48 h) |
|---|---|---|---|---|---|
| 4a | CH3 | 73.32 ± 3.28 c | 4l | (4-Cl)-benzyl | 72.46 ± 2.79 c |
| 4b | CH2CH3 | 78.26 ± 3.53 d,e | 4m | (2-CH3)-phenyl | 42.84 ± 3.09 a |
| 4c | (CH2)2CH3 | 86.49 ± 2.46 f,g | 4n | (3-CH3)-phenyl | 44.91 ± 1.74 a |
| 4d | (CH2)3CH3 | 83.22 ± 3.74 e,f | 4o | (4-CH3)-phenyl | 79.14 ± 2.69 e |
| 4e | (CH2)4CH3 | 80.18 ± 4.66 e | 4p | (4-CH2CH3)-phenyl | 80.89 ± 2.57 e |
| 4f | CH(CH3)2 | 73.85 ± 3.51 c,d | 4q | (4-F)-phenyl | 88.86 ± 4.71 g |
| 4g | CHCH2(CH3)2 | 70.63 ± 4.28 c | 4r | (4-Cl)-phenyl | 82.05 ± 3.74 e,f |
| 4h | C(CH3)3 | 63.26 ± 2.80 b | 4s | (4-OCH3)-phenyl | 80.83 ± 2.68 e |
| 4i | cyclohexyl | 61.57 ± 3.24 b | 4t | (2,4-(CH3)2)-phenyl | 74.05 ± 5.33 c,d |
| 4j | phenyl | 60.78 ± 3.17 b | EβF | - | 60.25 ± 4.42 b |
| 4k | benzyl | 59.69 ± 3.43 b | Pymetrozine | - | 80.68 ± 3.83 e |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Zhang, J.; Song, D.; Duan, H.; Li, W.; Yang, X. Novel (E)-β-Farnesene Analogues Containing 2-Nitroiminohexahydro-1,3,5-triazine: Synthesis and Biological Activity Evaluation. Molecules 2016, 21, 825. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21070825
Qin Y, Zhang J, Song D, Duan H, Li W, Yang X. Novel (E)-β-Farnesene Analogues Containing 2-Nitroiminohexahydro-1,3,5-triazine: Synthesis and Biological Activity Evaluation. Molecules. 2016; 21(7):825. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21070825
Chicago/Turabian StyleQin, Yaoguo, Jingpeng Zhang, Dunlun Song, Hongxia Duan, Wenhao Li, and Xinling Yang. 2016. "Novel (E)-β-Farnesene Analogues Containing 2-Nitroiminohexahydro-1,3,5-triazine: Synthesis and Biological Activity Evaluation" Molecules 21, no. 7: 825. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules21070825