Antigiardial Activity of Podophyllotoxin-Type Lignans from Bursera fagaroides var. fagaroides
Abstract
:1. Introduction
2. Results
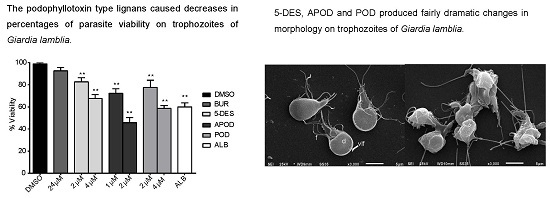
2.1. Dose-Dependent Effect of Podophyllotoxin-Type Lignans from Bursera fagaroides on Giardia lamblia Trophozoite Growth and Viability
2.2. Podophyllotoxin-Type Lignans Affect the Adhesion of Giardia lamblia Trophozoites
2.3. Podophyllotoxin-Type Lignans Affect the Morphology of Giardia lamblia Trophozoites
2.4. Cytotoxic Effect of Podophyllotoxin-Type Lignans on Human Intestinal Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Podophyllotoxin-Type Lignans from Bursera fagaroides var. fagaroides
4.2. Culture of Giardia lamblia
4.3. Cytotoxic Assay and Cell Viability
4.4. Adherence Inhibition Assays
4.5. The Effect of Podophyllotoxin-Type Lignans on Morphology by Scanning Electron Microscopy (SEM)
4.6. Culture of the Human Intestinal Caco-2 Cells
4.7. Cell Viability (MTT Assay)
4.8. Structure Similarity Analysis of Podophyllotoxin-Type Lignans
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adam, R.D. Biology of giardia lamblia. Clin. Microbiol. Rev. 2001, 14, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Almirall, P.; Nunez, F.A.; Bello, J.; Gonzalez, O.M.; Fernandez, R.; Escobedo, A.A. Abdominal pain and asthenia as common clinical features in hospitalized children for giardiasis. Acta Trop. 2013, 127, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, A.A.; Almirall, P.; Robertson, L.J.; Franco, R.M.; Hanevik, K.; Morch, K.; Cimerman, S. Giardiasis: The ever-present threat of a neglected disease. Infect. Disord Drug Targets 2010, 10, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Espelage, W.; an der Heiden, M.; Stark, K.; Alpers, K. Characteristics and risk factors for symptomatic giardia lamblia infections in germany. BMC Public Health 2010, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Jex, A.R.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. Giardia/giardiasis—A perspective on diagnostic and analytical tools. Biotechnol. Adv. 2014, 32, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ansell, B.R.; McConville, M.J.; Ma’ayeh, S.Y.; Dagley, M.J.; Gasser, R.B.; Svard, S.G.; Jex, A.R. Drug resistance in giardia duodenalis. Biotechnol. Adv. 2015, 33, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Granados, C.E.; Reveiz, L.; Uribe, L.G.; Criollo, C.P. Drugs for treating giardiasis. Cochrane Database Syst. Rev. 2012, 12, 1–52. [Google Scholar]
- Lalle, M. Giardiasis in the post genomic era: Treatment, drug resistance and novel therapeutic perspectives. Infect Disord Drug Targets 2010, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Leitsch, D. Drug resistance in the microaerophilic parasite. Curr. Trop. Med. Rep. 2015, 2, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Arellanes, A.; Cornejo-Garrido, J.; Rojas-Bribiesca, G.; Nicasio-Torres Mdel, P.; Said-Fernandez, S.; Mata-Cardenas, B.D.; Molina-Salinas, G.M.; Tortoriello, J.; Meckes-Fischer, M. Microbiological and pharmacological evaluation of the micropropagated rubus liebmannii medicinal plant. Evid. Based Complement Altern. Med. 2012, 2012, 503031. [Google Scholar] [CrossRef] [PubMed]
- Rayan, P.; Matthews, B.; McDonnell, P.A.; Cock, I.E. Terminalia ferdinandiana extracts as inhibitors of giardia duodenalis proliferation: A new treatment for giardiasis. Parasitol. Res. 2015, 114, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Rufino-Gonzalez, Y.; Ponce-Macotela, M.; Gonzalez-Maciel, A.; Reynoso-Robles, R.; Jimenez-Estrada, M.; Sanchez-Contreras, A.; Martinez-Gordillo, M.N. In vitro activity of the f-6 fraction of oregano against giardia intestinalis. Parasitology 2012, 139, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.; Miguel del Corral, J.M.; Gordaliza, M.; Garcia, P.A.; Gomez-Zurita, M.A.; Garcia-Gravalos, M.D.; de la Iglesia-Vicente, J.; Gajate, C.; An, F.; Mollinedo, F.; et al. Synthesis and biological evaluation of new selective cytotoxic cyclolignans derived from podophyllotoxin. J. Med. Chem. 2004, 47, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.; Garcia, P.A.; del Corral, J.M.; Castro, M.A.; Gomez-Zurita, M.A. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Ali Hussaini, S.M.; Rahim, A.; Riyaz, S. Podophyllotoxin derivatives: A patent review (2012–2014). Expert Opin. Ther. Pat. 2015, 25, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Fukuyama, M.; Yoshio, K.; Kato, T.; Ishikawa, Y. Biologically active components against drosophila melanogaster from podophyllum hexandrum. J. Agric. Food Chem. 1999, 47, 5108–5110. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, H.; Shahbazi, M.; Mohammadi, F.; Mohammadi, F. The anti-parasite effect of podophyllotoxin on giardia lamblia. Indian J. Sci. Res. 2014, 5, 310–313. [Google Scholar]
- Rzedowski, J.; Medina-Lemos, R.; de Rzedowski, G.C. Inventario del conocimiento taxonómico, así como de la diversidad y del endemismo regionales de las especies mexicanas de bursera (burseraceae). Acta Bot. Mex. 2005, 70, 85–111. [Google Scholar] [CrossRef]
- Acevedo, M.; Nuñez, P.; Gónzalez, L.; Cardozo-Taketa, A.; Villarreal, M. Cytotoxic and anti-inflammatory activities of bursera species from mexico. J. Clin. Toxicol. 2015, 5, 232. [Google Scholar]
- Morales-Serna, J.A.; Cruz-Galicia, E.; Garcia-Rios, E.; Madrigal, D.; Gavino, R.; Cardenas, J.; Salmon, M. Three new diarylbutane lignans from the resin of bursera fagaroides. Nat. Prod. Res. 2012, 27, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Reynoso-Orozco, R.; Santerre, A.; Delgado-Saucedo, J.I.; Solis, J.C.; Velazquez-Magaña, S.; Puebla-Perez, A.M. Polyamines as biomarkers of the antitumoral activity of bursera fagaroides. Interciencia 2008, 33, 384–388. [Google Scholar]
- Rojas-Sepulveda, A.M.; Mendieta-Serrano, M.; Mojica, M.Y.; Salas-Vidal, E.; Marquina, S.; Villarreal, M.L.; Puebla, A.M.; Delgado, J.I.; Alvarez, L. Cytotoxic podophyllotoxin-type-lignans from the steam bark of bursera fagaroides var. Fagaroides. Molecules 2012, 17, 9506–9519. [Google Scholar] [CrossRef] [PubMed]
- Puebla-Pérez, A.M.; Huacuja-Ruiz, L.; Rodríguez-Orozco, G.; Villaseñor-García, M.M.; Miranda-Beltrán, M.D.L.L.; Celis, A.; Sandoval-Ramírez, L. Cytotoxic and antitumour activity from bursera fagaroides ethanol extract in mice with l5178y lymphoma. Phytother. Res. 1998, 12, 545–548. [Google Scholar] [CrossRef]
- Rosas-Arreguin, P.; Arteaga-Nieto, P.; Reynoso-Orozco, R.; Villagomez-Castro, J.C.; Sabanero-Lopez, M.; Puebla-Perez, A.M.; Calvo-Mendez, C. Bursera fagaroides, effect of an ethanolic extract on ornithine decarboxylase (odc) activity in vitro and on the growth of entamoeba histolytica. Exp. Parasitol. 2008, 119, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Antunez-Mojica, M.; Leon, A.; Rojas-Sepulveda, A.M.; Marquina, S.; Mendieta-Serrano, M.A.; Salas-Vidal, E.; Villarreal, M.L.; Alvarez, L. Aryldihydronaphthalene-type lignans from bursera fagaroides var. Fagaroides and their antimitotic mechanism of action. RSC Adv. 2016, 6, 4950–4959. [Google Scholar] [CrossRef]
- Velazquez-Jimenez, R.; Torres-Valencia, J.M.; Cerda-Garcia-Rojas, C.M.; Hernandez-Hernandez, J.D.; Roman-Marin, L.U.; Manriquez-Torres, J.J.; Gomez-Hurtado, M.A.; Valdez-Calderon, A.; Motilva, V.; Garcia-Maurino, S.; et al. Absolute configuration of podophyllotoxin related lignans from bursera fagaroides using vibrational circular dichroism. Phytochemistry 2011, 72, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.B.; Hill, D.R. Treatment of giardiasis. Clin. Microbiol. Rev. 2001, 14, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, C.X.; Sun, H.; Jin, L.; Guo, C.Y.; Cao, W.; Wu, J.; Tian, H.Y.; Luo, C.; Ye, W.C.; et al. Protective effects and mechanisms of curcumin on podophyllotoxin toxicity in vitro and in vivo. Toxicol. Appl. Pharmacol. 2012, 265, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, H.; Jin, L.; Cao, W.; Zhang, J.; Guo, C.Y.; Ding, K.; Luo, C.; Ye, W.C.; Jiang, R.W. Alleviation of podophyllotoxin toxicity using coexisting flavonoids from dysosma versipellis. PLoS ONE 2013, 8, e72099. [Google Scholar] [CrossRef] [PubMed]
- Erlandsen, S.L.; Russo, A.P.; Turner, J.N. Evidence for adhesive activity of the ventrolateral flange in giardia lamblia. J. Eukaryot. Microbiol. 2004, 51, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Antúnez-Mojica, M.; Rodríguez-Salarichs, J.; Redondo-Horcajo, M.; León, A.; Barasoain, I.; Canales, Á.; Cañada, F.J.; Jimínez-Barbero, J.; Alvarez, L.; Díaz, J.F. Structural and biochemical characterization of the interaction of tubulin with potent natural analogues of podophyllotoxin. J. Nat. Prod. 2016, 79, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Meloni, B.P.; Thompson, R.C.; Reynoldson, J.A.; Seville, P. Albendazole: A more effective antigiardial agent in vitro than metronidazole or tinidazole. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 375–379. [Google Scholar] [CrossRef]
- Peña-Moran, O.A.; Villarreal, M.L.; Alvarez-Berber, L.; Meneses-Acosta, A.; Rodriguez-Lopez, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from bursera fagaroides var. Fagaroides on breast cancer cell lines. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Whittle, M.; Gillet, V.J.; Willett, P.; Alex, A.; Loesel, J. Enhancing the effectiveness of virtual screening by fusing nearest neighbor lists: A comparison of similarity coefficients. J. Chem. Inf. Comput. Sci. 2004, 44, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, T.T. An elementary mathematical theory of classification and prediction; Technical Report; International Business Machines Corporation: New York, NY, USA, 1958. [Google Scholar]
- Fligner, M.A.; Verducci, J.S.; Blower, P.E. A modification of the jaccard-tanimoto similarity index for diverse selection of chemical compounds using binary strings. Technometrics 2002, 44, 110–119. [Google Scholar] [CrossRef]
- Xu, H.; Lv, M.; Tian, X. A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003–2007. Curr. Med. Chem. 2009, 16, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Srinivasa Reddy, T.; Polepalli, S.; Shalini, N.; Reddy, V.G.; Subba Rao, A.V.; Jain, N.; Shankaraiah, N. Synthesis and biological evaluation of podophyllotoxin congeners as tubulin polymerization inhibitors. Bioorg. Med. Chem. 2014, 22, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fang, S.; Li, H.; Han, C.; Lu, Y.; Zhao, Y.; Liu, Y.; Zhao, C. Biological evaluation and molecular modelling study of podophyllotoxin derivatives as potent inhibitors of tubulin polymerization. Chem. Biol. Drug Des. 2013, 82, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, V.; Mastronicola, D.; Singh, A.K.; Tiwari, H.K.; Pucillo, L.P.; Sarti, P.; Singh, B.K.; Giuffré, A. Antigiardial activity of novel triazolyl-quinolone-based chalcone derivatives: When oxygen makes the difference. Front. Microbiol. 2015, 6, 256. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| Lignan | Giardia IC50 µM | Caco-2 IC50 µM | Selectivity (IC50 Caco-2/IC50 Giardia) |
|---|---|---|---|
| Burseranin | 42.22 | 19.69 | 0.47 |
| 5′-demethoxy-β-peltatin-A-methylether | 4.53 | 2.87 | 0.62 |
| Acetylpodophyllotoxin | 2.12 | 8.64 | 4.1 |
| Podophyllotoxin | 3.88 | 0.65 | 0.15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Gutiérrez, F.; Puebla-Pérez, A.M.; González-Pozos, S.; Hernández-Hernández, J.M.; Pérez-Rangel, A.; Alvarez, L.P.; Tapia-Pastrana, G.; Castillo-Romero, A. Antigiardial Activity of Podophyllotoxin-Type Lignans from Bursera fagaroides var. fagaroides. Molecules 2017, 22, 799. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050799
Gutiérrez-Gutiérrez F, Puebla-Pérez AM, González-Pozos S, Hernández-Hernández JM, Pérez-Rangel A, Alvarez LP, Tapia-Pastrana G, Castillo-Romero A. Antigiardial Activity of Podophyllotoxin-Type Lignans from Bursera fagaroides var. fagaroides. Molecules. 2017; 22(5):799. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050799
Chicago/Turabian StyleGutiérrez-Gutiérrez, Filiberto, Ana María Puebla-Pérez, Sirenia González-Pozos, José Manuel Hernández-Hernández, Armando Pérez-Rangel, Laura Patricia Alvarez, Gabriela Tapia-Pastrana, and Araceli Castillo-Romero. 2017. "Antigiardial Activity of Podophyllotoxin-Type Lignans from Bursera fagaroides var. fagaroides" Molecules 22, no. 5: 799. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22050799