Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride
Abstract
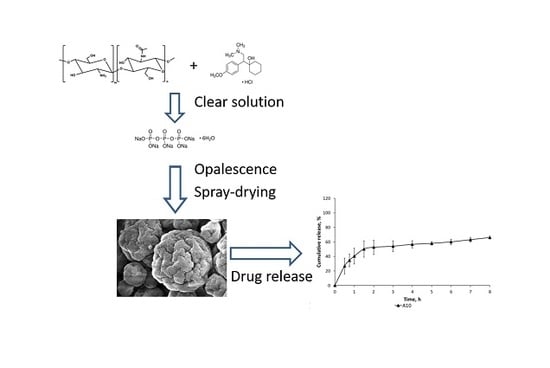
:1. Introduction
2. Results and Discussion
2.1. Chitosan Characterization
2.2. Microcapsules Characterization
2.3. Drug Physical State
2.4. In Vitro Drug Release in Simulated Gastric Fluid (SGF) and Simulated Intestinal Fluid (SIF)
- (i)
- Due to water imbibition, chitosan swells increasing the dimensions of the particles. This swelling is controlled by the polymer cross-linking with TPP.
- (ii)
- Upon contact with water, venlafaxine dissolves and, due to concentration gradients, diffuses out of the microcapsules.
- (iii)
- Depending on microcapsule composition, certain polymer dissolution in SGF is expected. Since microcapsules maintain their integrity during the release assay even in the absence of TPP this phenomenon may be negligible at least at the beginning of release.
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Chitosan Deacetylation Degree Determination
3.2.2. Determination of Intrinsic Viscosity ([η]) and Molar Mass Estimation of Chitosans
3.2.3. Microcapsules Production
3.2.4. Spray Drying Production Yield
3.2.5. Encapsulation Efficiency
3.2.6. Shape and Morphology Determination
3.2.7. Size Distribution
3.2.8. Zeta Potential
3.2.9. X-ray Diffraction (XRD) Studies
3.2.10. In Vitro Release of Venlafaxine
3.2.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Broquet, K.E. Status of treatment of depression. South. Med. J. 1999, 92, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Troy, S.M.; Parker, V.D.; Fruncillo, R.J.; Chiang, S.T. The pharmacokinetics of venlafaxine when given in a twice-daily regimen. J. Clin. Pharmacol. 1995, 35, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.A.; Shah, S.; Shah, D.O.; Joshi, P.A. Sustained release of venlafaxine from venlafaxine–montmorillonite–polyvinylpyrrolidone composites. Appl. Clay Sci. 2011, 51, 126–130. [Google Scholar] [CrossRef]
- Jain, S.; Datta, M. Montmorillonite-PLGA nanocomposites as an oral extended drug delivery vehicle for venlafaxine hydrochloride. Appl. Clay Sci. 2014, 99, 42–47. [Google Scholar] [CrossRef]
- Jain, S.; Datta, M. Montmorillonite-alginate microspheres as a delivery vehicle for oral extended release of venlafaxine hydrochloride. J. Drug Deliv. Sci. Technol. 2016, 33, 149–156. [Google Scholar] [CrossRef]
- Ajit, I.; Senthil, A.; Rahul, B.; Narayanaswamy, V.B. Formulation and evaluation of venlafaxine hydrochloride mucoadhesive buccal tablets. Int. Res. J. Pharm. 2012, 3, 226–231. [Google Scholar]
- Yadav, A.V.; Urade, M.N. Preparation and Evaluation of Chitosan Containing Mucoadhesive Buccal Films of Venlafaxine Hydrochloride. Res. J. Pharm. Technol. 2010, 3, 1213–1217. [Google Scholar]
- Peng, Y.; Li, J.; Li, J.; Fei, Y.; Dong, J.; Pan, W. Optimization of thermosensitive chitosan hydrogels for the sustained delivery of venlafaxine hydrochloride. Int. J. Pharm. 2013, 441, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengıbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohyd. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopeia 6.0 1774. Chitosan Hydrochloride; Council of Europe: Strarsburg, Germany, 2008. [Google Scholar]
- United States Pharmacopeial Convection. United States Pharmacopeia 34/National Formulary. Chitosan; The United States Pharmacopeial Convection: Rockville, MD, USA, 2011. [Google Scholar]
- Masters, K. The Spray Drying Handbook; Longman Scientific and Technical: New York, NY, USA, 1991. [Google Scholar]
- Fu, Y.J.; Mi, F.L.; Wong, T.B.; Shyu, S.S. Characteristic and controlled release of anticancer drug loaded poly (d,l-lactide) microparticles by spray drying technique. J. Microencapsul. 2001, 18, 733–747. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Davis, S.S.; Illum, L. Chitosan microspheres prepared by spray drying. Int. J. Pharm. 1999, 187, 53–65. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Preparation and Characterization of Drug-Loaded Chitosan–Tripolyphosphate Microspheres by Spray Drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Database of Select Committee on GRAS Substances (SCOGS) Reviews; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2006.
- The European Parliament and of the Council of 18 March 1995 on Food Additives Other than Colours and Sweeteners (OJ L 61, 18.3.1995, p. 1) as Successively Amended. Directive 95/2/EC. 1995.
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Henriksen, I.; Green, K.L.; Smart, J.D.; Smitad, G.; Karlsen, J. Bioadhesion of hydrated chitosan: An in vitro and an in vivo study. Int. J. Pharm. 1996, 145, 231–240. [Google Scholar] [CrossRef]
- Soane, R.J.; Frier, M.; Perkins, A.C.; Jones, N.S.; Davis, S.S.; Illum, L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 1999, 178, 55–65. [Google Scholar] [CrossRef]
- Shah, S.; Pal, A.; Kaushik, V.K.; Devi, S. Preparation and Characterization of Venlafaxine Hydrochloride-Loaded Chitosan Nanoparticles and In Vitro Release of Drug. J. Appl. Polym. Sci. 2009, 112, 2876–2887. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Rohani, S.S.R.; Abnous, K.; Tafaghodi, M. Preparation and characterization of spray-dried powders intended for pulmonary delivery of Insulin with regard to the selection of excipients. Int. J. Pharm. 2014, 465, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Nerkar, P.; Gattani, S. Spray-dried buccal mucoadhesive microparticles of venlafaxine based on cress seed mucilage: In vitro, in vivo evaluation in rabbits. Dry. Technol. 2012, 30, 968–978. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Bari, D.B.; Surana, S.J.; Pardeshia, C V. In vitro, ex vivo and in vivo performance of chitosan-based spray-dried nasal mucoadhesive microspheres of diltiazem hydrochloride. J. Drug Deliv. Sci. Technol. 2016, 31, 108–117. [Google Scholar] [CrossRef]
- Anal, A.K.; Stevens, W.F.; Remuñán-López, C. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int. J. Pharm. 2006, 312, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.S.; Oliveira, P.R.; Murakami, F.S.; Borgmann, S.H.M.; Arend, M.Z.; Cardoso, S.G. Development and Validation of a Stability-Indicating LC Method for the Determination of Venlafaxine in Extended-Release Capsules and Dissolution Kinetic Studies. J. Chromatogr. Sci. 2009, 47, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Rinaki, E.; Valsami, G.; Macheras, P. The power law can describe the ‘entire’ drug release curve from HPMC-based matrix tablets: A hypothesis. Int. J. Pharm. 2003, 255, 199–207. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Surí, J.; Millán, D.; Suñé-Negre, J.M.; Colom, H.; Ticó, J.R.; Miñarro, M.; Pérez-Lozano, P.; García-Montoya, E. Quality by design approach to understand the physicochemical phenomena involved in controlled release of captopril SR matrix tablets. Int. J. Pharm. 2014, 477, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.A.; Rocchetti, R.; Stanic, V.; Weckx, M. Methods for the determination of the degree of acetylation of chitin and chitosan. In Chitin Handbook; Muzzarelli, R.A.A., Peter, M.G., Eds.; European Chitin Society: Seville, Italy, 1997; pp. 109–115. ISBN 13 9788886889018. [Google Scholar]
- Rinaudo, M.; Milas, M.; LeDung, P. Characterization of chitosan. Influence of ionic strength and degree of acetylation on chain expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Hildebrand, G.E.; Nitzsche, R.; Paulke, B.R. Zetapotential. In Zetapotential und Partikelladung in der Laborpraxis; Wissenschaftliche Verlagsgesellchaft GmbH: Stuttgart, Germany, 1996; pp. 19–99. ISBN 13 9783804714656. [Google Scholar]
- United States Pharmacopeial Convection. United States Pharmacopeia 23th revision (USP 23/NF 18), 12601; Twinbrook Parkway: Rockville, MD, USA, 1995. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |






| Sample Name | Source | Ash Content, % | (η), dL/g | Mv, kDa | DD, % |
|---|---|---|---|---|---|
| CS-1 | Blue Crab (Callinectes sp.) | 0.01 | 1.975 | 644 | 90.5 |
| CS-2 | King Crab (Paralomis granulosa) | <0.01 | 1.171 | 324 | 83.8 |
| Formulation Code | CS Sample | [TPP], % | CS:TPP | Practical Yield, % | EE, % | ζ, mV |
|---|---|---|---|---|---|---|
| A1 | CS-1 | 0 | - | 63 | 56 ± 3 | +26.6 |
| A2 | CS-2 | 0 | - | 53 | 88 ± 3 | +19.1 |
| A3 | CS-1 | 0.1 | 100:30 | 44 | 81 ± 2 | +21.5 |
| A4 | CS-2 | 0.1 | 100:30 | 65 | 94 ± 1 | +16.6 |
| A5 | CS-1 | 0.2 | 100:30 | 45 | 76 ± 3 | +13.4 |
| A6 | CS-2 | 0.2 | 100:30 | 62 | 90 ± 3 | +16.6 |
| A7 | CS-1 | 0.5 | 100:30 | 55 | 51 ± 5 | +1.3 |
| A8 | CS-2 | 0.5 | 100:30 | 62 | 66 ± 3 | −0.8 |
| A9 | CS-2 | 0.5 | 100:80 | 64 | 37 ± 3 | −5.7 |
| A10 | CS-2 | 0.5 | 100:100 | 74 | 38 ± 4 | −9.4 |
| Formulation Code | n SGF | SSD | n SIF | SSD |
|---|---|---|---|---|
| A1 | 0.63 | 0.00027 | 0.83 | 0.007998 |
| A2 | 0.49 | 0.00246 | 0.71 | 0.001473 |
| A3 | 0.69 | 0.00576 | 0.75 | 0.000464 |
| A4 | 0.70 | 0.00093 | 0.68 | 0.000154 |
| A5 | 0.66 | 0.00629 | 0.55 | 0.002309 |
| A6 | 0.84 | 0.00415 | 0.47 | 0.000002 |
| A7 | 0.56 | 0.00028 | 0.54 | 0.000001 |
| A8 | 0.71 | 0.00001 | 0.55 | 0.000292 |
| A9 | 0.60 | 0.00007 | 0.62 | 0.000857 |
| A10 | 0.55 | 0.00328 | 0.32 | 0.003435 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranaz, I.; Paños, I.; Peniche, C.; Heras, Á.; Acosta, N. Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride. Molecules 2017, 22, 1980. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111980
Aranaz I, Paños I, Peniche C, Heras Á, Acosta N. Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride. Molecules. 2017; 22(11):1980. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111980
Chicago/Turabian StyleAranaz, Inmaculada, Ines Paños, Carlos Peniche, Ángeles Heras, and Niuris Acosta. 2017. "Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride" Molecules 22, no. 11: 1980. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules22111980