Hierarchical Self-Assembled Structures from Diblock Copolymer Mixtures by Competitive Hydrogen Bonding Strength
Abstract
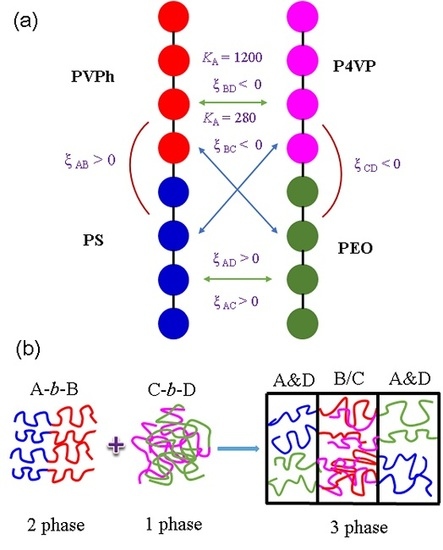
:1. Introduction
2. Results and Discussion
2.1. The Preparation of PS-b-PVPh and PEO-b-P4VP Diblock Copolymers
2.2. Self-Assembled Structure of PS-b-PVPh/PEO Diblock Copolymer/Homopolymer Mixtures
2.3. Self-Assembled Structure of PS-b-PVPh/PEO-b-P4VP Diblock Copolymer Mixtures
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. The Synthesis of PEO-b-P4VP Diblock Copolymer by Using RAFT Polymerization
4.3. PS-b-PVPh/PEO-b-P4VP Blends
4.4. Characterizations
Author Contributions
Funding
Conflicts of Interest
References
- Jenekhe, S.A.; Chen, X.L. Self-assembly of ordered microporous materials from rod-coil block copolymers. Science 1999, 283, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Guiod, A.R.; Vandermeulen, W.M.; Klok, V.H. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar]
- Lin, E.L.; Hsu, W.L.; Chiang, Y.W. Trapping structural coloration by a bioinspired gyroid microstructure in solid state. ACS Nano 2018, 12, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.T.; Chiu, H.W.; Nababan, R.; Le, Q.M.; Kuo, S.W.; Chau, L.K.; Ting, C.C.; Kan, H.C.; Hsu, C.C. Enhancing upconversion luminescence emission of rare earth nanophosphors in aqueous solution with thousands fold enhancement factor by low refractive index resonant waveguide grating. ACS Photonics 2018, 5, 3263–3271. [Google Scholar] [CrossRef]
- Jiang, M.; Xie, H. Miscibility and morphology in block copolymer/homopolymer blends. Prog. Polym. Sci. 1991, 16, 977–1026. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Pearce, E.M.; Kwei, T.K. Binary and ternary blends of polystyrene-block-poly(p-hydroxystyrene). Macromolecules 1997, 30, 7119–7126. [Google Scholar] [CrossRef]
- Han, Y.K.; Pearce, E.M.; Kwei, T.K. Poly(styrene-b-vinylphenyldimethylsilanol) and its blends with homopolymers. Macromolecules 2000, 33, 1321–1329. [Google Scholar] [CrossRef]
- Kosoneen, H.; Ruokolainen, J.; Nyholm, P.; Ikkala, O. Self-organized cross-linked phenolic thermosets: Thermal and dynamic mechanical properties of novolac/block copolymer blends. Polymer 2001, 42, 9481–9486. [Google Scholar] [CrossRef]
- Dobrosielska, K.; Wakao, S.; Takano, A.; Matsushita, Y. Nanophase-separated structures of AB block copolymer/C homopolymer blends with complementary hydrogen-bonding interactions. Macromolecules 2008, 41, 7695–7698. [Google Scholar] [CrossRef]
- Dobrosielska, K.; Wakao, S.; Suzuki, J.; Noda, K.; Takano, A.; Matsushita, Y. Effect of homopolymer molecular weight on nanophase-separated structures of AB block copolymer/C homopolymer blends with hydrogen-bonding interactions. Macromolecules 2009, 42, 7098–7102. [Google Scholar] [CrossRef]
- Chen, S.C.; Kuo, S.W.; Jeng, U.S.; Su, C.J.; Chang, F.C. On modulating the phase behavior of block copolymer/homopolymer blends via hydrogen bonding. Macromolecules 2010, 43, 1083–1092. [Google Scholar] [CrossRef]
- Dehghan, A.; Shi, A.C. Modeling hydrogen bonding in diblock copolymer/homopolymer blends. Macromolecules 2013, 46, 5796–5805. [Google Scholar] [CrossRef]
- Tsai, S.C.; Lin, Y.C.; Lin, E.L.; Chiang, Y.W.; Kuo, S.W. Hydrogen bonding strength effect on self-assembly supramolecular structures of diblock copolymer/homopolymer blends. Polym. Chem. 2016, 7, 2395–2409. [Google Scholar] [CrossRef]
- Hameed, N.; Guo, Q. Nanostructure and hydrogen bonding in interpolyelectrolyte complexes of poly(ɛ-caprolactone)-block-poly(2-vinyl pyridine) and poly(acrylic acid). Polymer 2008, 49, 5268–5275. [Google Scholar] [CrossRef]
- Hameed, N.; Liu, J.; Guo, Q. Self-assembled complexes of poly(4-vinylphenol) and poly(ε-caprolactone)-block-poly (2-vinylpyridine) via competitive hydrogen bonding. Macromolecules 2008, 41, 7596–7605. [Google Scholar] [CrossRef]
- Hameed, N.; Guo, Q. Selective hydrogen bonding and hierarchical nanostructures in poly(hydroxyether of bisphenol A)/poly(ɛ-caprolactone)-block-poly(2-vinyl pyridine) blends. Polymer 2008, 49, 922–933. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W.; Lu, C.H.; Jeng, U.S.; Chang, F.C. Self-assembly structures through competitive interactions of crystalline−amorphous diblock copolymer/homopolymer blends: Poly(ε-caprolactone-b-4-vinyl pyridine)/poly(vinyl phenol). Macromolecules 2009, 42, 3580–3590. [Google Scholar] [CrossRef]
- Salim, N.V.; Hanley, T.; Guo, Q. Microphase separation through competitive hydrogen bonding in double crystalline diblock copolymer/homopolymer blends. Macromolecules 2010, 43, 7695–7704. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, Y.D.; Kuo, S.W. From microphase separation to self-organized mesoporous phenolic resin through competitive hydrogen bonding with double-crystalline diblock copolymers of poly(ethylene oxide-b-ε-caprolactone). Macromolecules 2011, 44, 9295–9309. [Google Scholar] [CrossRef]
- Salim, N.V.; Hameed, N.; Guo, Q. Competitive hydrogen bonding and self-assembly in poly(2-vinyl pyridine)-block-poly(methyl methacrylate)/poly(hydroxyether of bisphenol A) blends. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1894–1905. [Google Scholar] [CrossRef]
- Hameed, N.; Salim, N.V.; Guo, Q. Microphase separation through competitive hydrogen bonding in self-assembled diblock copolymer/homopolymer complexes. J. Chem. Phys. 2009, 131, 214905. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.F.; Kuo, S.W.; Huang, C.F.; Lu, J.S.; Chan, S.C.; Wang, C.F.; Chang, F.C. Hydrogen-bonding interactions mediate the phase behavior of an AB/C block copolymer/homopolymer blend comprising poly (methyl methacrylate-b-vinylpyrrolidone) and poly (vinylphenol). Macromolecules 2006, 39, 5458–5465. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W.; Jeng, U.S.; Chang, F.C. Self-assembly through competitive interactions of miscible diblock copolymer/homopolymer blends: Poly (vinylphenol-b-methyl methacrylate)/poly (vinylpyrrolidone) blend. Macromolecules 2008, 41, 1401–1410. [Google Scholar] [CrossRef]
- Zhou, J.; Shi, A.C. Microphase separation induced by differential interactions in diblock copolymer/homopolymer blends. J. Chem. Phys. 2009, 130, 234904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, I.; Kuo, S.W.; Chang, F.C. Self-Assembly structures through competitive interactions of miscible crystalline–amorphous diblock copolymer/homopolymer blends. Polymer 2009, 50, 5276–5287. [Google Scholar] [CrossRef]
- Matsushita, Y. Creation of hierarchically ordered nanophase structures in block polymers having various competing interactions. Macromolecules 2007, 40, 771–776. [Google Scholar] [CrossRef]
- Miyase, H.; Asai, Y.; Takano, A.; Matsushita, Y. Kaleidoscopic tiling patterns with large unit cells from ABC star-shaped terpolymer/diblock copolymer blends with hydrogen bonding interaction. Macromolecules 2017, 50, 979–986. [Google Scholar] [CrossRef]
- Asari, T.; Matsuo, S.; Takano, A.; Matsushita, Y. Three-phase hierarchical structures from AB/CD diblock copolymer blends with complemental hydrogen bonding interaction. Macromolecules 2005, 38, 8811–8815. [Google Scholar] [CrossRef]
- Asari, T.; Arai, S.; Takano, A.; Matsushita, Y. Archimedean tiling structures from ABA/CD block copolymer blends having intermolecular association with hydrogen bonding. Macromolecules 2006, 39, 2232–2237. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen bond-mediated self-assembly and supramolecular structures of diblock copolymer mixtures. Polym. Int. 2009, 58, 455–464. [Google Scholar] [CrossRef]
- Chen, W.C. , Kuo, S.W., Chang, F.C. Self-assembly of an A–B diblock copolymer blended with a C homopolymer and a C–D diblock copolymer through hydrogen bonding interaction. Polymer 2010, 51, 4176–4184. [Google Scholar] [CrossRef]
- Kuo, S.W.; Tung, P.H.; Lai, C.L.; Jeong, K.U.; Chang, F.C. Supramolecular micellization of diblock copolymer mixtures mediated by hydrogen bonding for the observation of separated coil and chain aggregation in common solvents. Macromol. Rapid Commun. 2008, 29, 229–233. [Google Scholar] [CrossRef]
- Kuo, S.W.; Tung, P.H.; Chang, F.C. Hydrogen bond mediated supramolecular micellization of diblock copolymer mixture in common solvents. Eur. Polym. J. 2009, 45, 1924–1935. [Google Scholar] [CrossRef]
- Hsu, C.H.; Kuo, S.W.; Chen, J.K.; Ko, F.H.; Liao, C.S.; Chang, F.C. Self-assembly behavior of AB diblock and CD random copolymer mixtures in the solution state through mediated hydrogen bonding. Langmuir 2008, 24, 7727–7734. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Lennon, E.M.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. Evolution of block copolymer lithography to highly ordered square arrays. Science 2008, 322, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Gan, Z.; Chen, T.; Kuo, S.W. Competitive Hydrogen Bonding Interactions Influence the Secondary and Hierarchical Self-Assembled Structures of Polypeptide-Based Triblock Copolymers. Macromolecules 2108, 51, 3017–3029. [Google Scholar] [CrossRef]
- Liu, C.C.; Chu, W.C.; Li, J.G.; Kuo, S.W. Mediated Competitive Hydrogen Bonding Form Mesoporous Phenolic Resins Templated by Poly (ethylene oxide-b-ε-caprolactone-b-l-lactide) Triblock Copolymers. Macromolecules 2014, 47, 6389–6400. [Google Scholar] [CrossRef]
- Tsai, C.C.; Gan, Z.; Kuo, S.W. Functional Porous Polypeptide with Benzoxazine Chemistry from Bio-based Triblock Copolymer for Efficient Dye Adsorption. Polym. Chem. 2108, 9, 3684–3693. [Google Scholar] [CrossRef]
- Tseng, T.C.; Kuo, S.W. Hydrogen-Bonding Strength Influences Hierarchical Self-Assembled Structures in Unusual Miscible/Immiscible Diblock Copolymer Blends. Macromolecules 2018, 51, 6451–6459. [Google Scholar] [CrossRef]
- Tung, P.H.; Kuo, S.W.; Chen, S.C.; Lin, C.L.; Chang, F.C. Micellar Morphologies of Self-Associated Diblock Copolymers in Acetone Solution. Polymer 2007, 48, 3192–3200. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lin, C.L.; Chang, F.C. Phase Behavior and Hydrogen Bonding in Ternary Polymer Blends of Phenolic Resin/Poly(ethylene oxide)/Poly(ε-caprolactone). Macromolecules 2002, 35, 278–285. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen-bonding in polymer blends. J. Polym. Res. 2008, 15, 459–486. [Google Scholar] [CrossRef]
- Kuo, S.W. Hydrogen Bonding in Polymeric Materials; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Coleman, M.M.; Painter, P.C. Miscible Polymer Blends: Background and Guide for Calculations and Design; DEStech Publication Inc.: Lancaster, PA, USA, 2006. [Google Scholar]
- Kuo, S.W.; Tung, P.H.; Chang, F.C. Syntheses and the study of strongly hydrogen-bonded poly (vinylphenol-b-vinylpyridine) diblock copolymer through anionic polymerization. Macromolecules 2006, 39, 9388–9395. [Google Scholar] [CrossRef]
- Yeh, C.L.; Hou, T.; Chen, H.L.; Yeh, L.Y.; Chiu, F.C.; Muller, A.J.; Hadjichristidis, N. Lower Critical Ordering Transition of Poly(ethyleneoxide)-block-poly(2-vinylpyridine). Macromolecules 2011, 44, 440–443. [Google Scholar] [CrossRef]
- Kwei, T.K. The Effect of Hydrogen Bonding on the Glass Transition Temperatures of Polymer Mixtures. J. Polym. Sci. Polym. Lett. Ed. 1984, 22, 307–313. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of PEO-b-P4VP and PS-b-PVPh are available from the authors. |













| Polymer | Molar Volume | Molecular Weight | Solubility Parameter | Equilibrium Constants | ||
|---|---|---|---|---|---|---|
| (mL/mol) | (g/mol) | ((cal/mL)1/2) | K2 | KB | KA | |
| PVPh | 82.3 | 120.1 | 11.0 | 21.0 | 66.8 | |
| PS | 93.9 | 104.1 | 9.5 | – | – | – |
| PEO | 38.1 | 44.1 | 9.40 | – | – | 280 |
| P4VP | 84.9 | 105.1 | 10.8 | – | – | 1200 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, T.-C.; Kuo, S.-W. Hierarchical Self-Assembled Structures from Diblock Copolymer Mixtures by Competitive Hydrogen Bonding Strength. Molecules 2018, 23, 2242. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092242
Tseng T-C, Kuo S-W. Hierarchical Self-Assembled Structures from Diblock Copolymer Mixtures by Competitive Hydrogen Bonding Strength. Molecules. 2018; 23(9):2242. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092242
Chicago/Turabian StyleTseng, Tzu-Chun, and Shiao-Wei Kuo. 2018. "Hierarchical Self-Assembled Structures from Diblock Copolymer Mixtures by Competitive Hydrogen Bonding Strength" Molecules 23, no. 9: 2242. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092242