Design, Synthesis and Biological Evaluation of Novel 4-Substituted Coumarin Derivatives as Antitumor Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Evaluation
- (i)
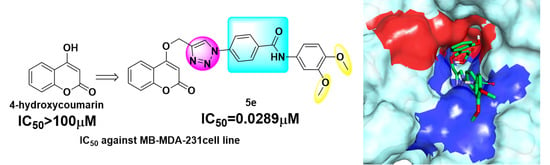
- All compounds showed better inhibition than that of 4-hydroxycoumarin and 5e had the best antiproliferative activity of this series of compounds, even better inhibition than that of DOX and cis-Pt under hypoxia;
- (ii)
- The IC50 of most compounds under hypoxic conditions were lower than that under normoxic conditions;
- (iii)
- The IC50, normoxia/IC50, hypoxia of DOX and cis-Pt were lower than that of 5b, 5e, 5h, 5m, 5o;
- (iv)
- The presence of a 4-substitued phenyl in the R group enhances the antiproliferative potential and antiproliferative activity increases remarkably when a 3,4-substitued phenyl is present in the R group.
2.3. Molecular Docking
3. Materials and Methods
3.1. Chemistry
3.1.1. 4-(Prop-2-ynyloxy)-2H-chromen-2-one (1)
3.1.2. 4-Azidobenzoic acid (2)
3.1.3. 4-(4-(((2-Oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)benzoic acid (3)
3.1.4. Synthesis of Compound 4
3.1.5. General Procedure for the Synthesis of Compound 5a–5e
3.2. In-Vitro Cytotoxicity Study (MTT Assay)
3.3. Molecular Docking Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shi, Y.; Zhou, C.H. Synthesis and evaluation of a class of new coumarin triazole derivatives as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Beillerot, A.; Domínguez, J.C.R.; Kirsch, G.; Bagrel, D. Synthesis and protective effects of coumarin derivatives against oxidative stress induced by doxorubicin. Bioorg. Med. Chem. Lett. 2008, 18, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, X.; Xu, W.; Farzaneh, F.; Xu, R. The Structure and Pharmacological Functions of Coumarins and Their Derivatives. Curr. Med. Chem. 2009, 16, 4236–4260. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, L.; Cavalli, A.; Colizzi, F.; Belluti, F.; Bartolini, M.; Mancini, F.; Recanatini, M.; Andrisano, V.; Rampa, A. Multi-target-directed coumarin derivatives: HAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg. Med. Chem. Lett. 2008, 18, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Fukuda, T.; Ishibashi, F.; Iwao, M. The first total synthesis of lamellarin α 20-sulfate, a selective inhibitor of HIV-1 integrase. Tetrahedron Lett. 2006, 47, 3755–3757. [Google Scholar] [CrossRef] [Green Version]
- Creaven, B.S.; Egan, D.A.; Karcz, D.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterisation and antimicrobial activity of copper(II) and manganese(II) complexes of coumarin-6,7-dioxyacetic acid (cdoaH2) and 4-methylcoumarin-6,7-dioxyacetic acid (4-MecdoaH2): X-ray crystal structures of [Cu(cdoa)(phen)2]·8.8H2O and [Cu(4-Mecdoa)(phen)2]·13H2O (phen=1,10-phenanthroline). J. Inorg. Biochem. 2007, 101, 1108–1119. [Google Scholar] [PubMed]
- Creaven, B.S.; Devereux, M.; Karcz, D.; Kellett, A.; McCann, M.; Noble, A.; Walsh, M. Copper(II) complexes of coumarin-derived Schiff bases and their anti-Candida activity. J. Inorg. Biochem. 2009, 103, 1196–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basanagouda, M.; Jambagi, V.B.; Barigidad, N.N.; Laxmeshwar, S.S.; Devaru, V. Narayanachar Synthesis, structure-activity relationship of iodinated-4-aryloxymethyl-coumarins as potential anti-cancer and anti-mycobacterial agents. Eur. J. Med. Chem. 2014, 74, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Le Lain, R.; Barrell, K.J.; Saeed, G.S.; Nicholls, P.J.; Simons, C.; Kirby, A.; Smith, H.J. Some coumarins and triphenylethene derivatives as inhibitors of human testes microsomal 17β-hydroxysteroid dehydrogenase (17β-HSD type 3): Further studies with tamoxifen on the rat testes microsomal enzyme. J. Enzyme Inhib. Med. Chem. 2002, 17, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Z.; Zhou, M.; Wu, F.; Hou, X.; Luo, H.; Liu, H.; Han, X.; Yan, G.; Ding, Z.; et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014, 24, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Patiar, S.; Supuran, C.T.; Harris, A.L.; Vaughan-Jones, R.D. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. J. Biol. Chem. 2009, 284, 20299–20310. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Supuran, C. Coumarins incorporating hydroxy-and chloro-moieties selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II. Bioorg. Med. Chem. Lett. 2010, 20, 4511–4514. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Cai, X.; Chennamaneni, S.; Yi, X.; Liu, L.; Pink, J.J.; Dowlati, A.; Xu, Y.; Zhou, A.; Su, B. From COX-2 inhibitor nimesulide to potent anti-cancer agent: Synthesis, in vitro, in vivo and pharmacokinetic evaluation. Eur. J. Med. Chem. 2012, 47, 432–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.M.; Huang, Z.N.; Zhou, Z.S.; Hou, J.; Zheng, M.Y.; Wang, L.J.; Jiang, Y.; Zhou, X.Y.; Chen, Q.Y.; Li, S.-H.; et al. Structure-based design, structure–activity relationship analysis, and antitumor activity of diaryl ether derivatives. Arch. Pharm. Res. 2015, 38, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Saekee, A.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, biological evaluation and molecular docking of novel chalcone-coumarin hybrids as anticancer and antimalarial agents. Eur. J. Med. Chem. 2014, 85, 65–76. [Google Scholar] [CrossRef] [PubMed]
- MatwiJczuk, A.; Janik, E.; Luchowski, R.; Niewiadomy, A.; Gruszecki, W.I.; Gagoś, M. Spectroscopic studies of the molecular organization of 4-([1,2,4] triazolo [4,3-a] pyridin-3-yl)-6-methylbenzene-1,3-diol in selected solvents. J. Lumin. 2018, 194, 208–218. [Google Scholar] [CrossRef]
- Singh, H.; Singh, J.V.; Gupta, M.K.; Saxena, A.K.; Sharma, S.; Nepali, K.; Bedi, P.M.S. Triazole tethered isatin-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies. Bioorg. Med. Chem. Lett. 2017, 27, 3974–3979. [Google Scholar] [CrossRef] [PubMed]
- Gieling, R.G.; Babur, M.; Mamnani, L.; Burrows, N.; Telfer, B.A.; Carta, F.; Winum, J.Y.; Scozzafava, A.; Supuran, C.T.; Williams, K.J. Antimetastatic effect of sulfamate carbonic anhydrase IX inhibitors in breast carcinoma xenografts. J. Med. Chem. 2012, 55, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Scozzafava, A.; Supuran, C.T. 7,8-Disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones i and II in the low nanomolar/subnanomolar range. Bioorg. Med. Chem. Lett. 2010, 20, 7255–7258. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.V.; Siddiqui, S.; SeiJas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; NikalJe, A.P.G. Microwave-assisted facile synthesis, anticancer evaluation and docking study of N-((5-(substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl) benzamide derivatives. Molecules 2017, 22, 995. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Temperini, C.; Vu, H.; Pham, N.B.; Poulsen, S.A.; Scozzafava, A.; Quinn, R.J.; Supuran, C.T. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J. Am. Chem. Soc. 2009, 131, 3057–3062. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hou, Z.; Tian, Y.; Mou, Y.; Guo, C. Design, synthesis, cytotoxicity and mechanism of novel dihydroartemisinin-coumarin hybrids as potential anti-cancer agents. Eur. J. Med. Chem. 2018, 151, 434–449. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 5a–5e are available from the authors. |






| Compd. | R | IC50 (Μm) a | IC50, normoxia/IC50, hypoxia b | |
|---|---|---|---|---|
| Hypoxia | Normoxic | |||
| 5a |  | 23.47 | 24.41 | 1.04 |
| 5b |  | 8.14 | 108.72 | 13.36 |
| 5c |  | 75.21 | 73.77 | 0.98 |
| 5d |  | 6.72 | 6.78 | 1.01 |
| 5e |  | 0.03 | 1.34 | 46.31 |
| 5f |  | 73.82 | 91.61 | 1.24 |
| 5g |  | 53.98 | 62.79 | 1.16 |
| 5h |  | 3.44 | 18.45 | 5.36 |
| 5i |  | 20.35 | 20.98 | 1.03 |
| 5j |  | 12.87 | 12.28 | 0.95 |
| 5k |  | 8.70 | 10.86 | 1.25 |
| 5l |  | 1.30 | 7.03 | 5.39 |
| 5m |  | 0.25 | 5.06 | 20.46 |
| 5n |  | 34.82 | 39.58 | 1.14 |
| 5o |  | 9.42 | 16.76 | 1.78 |
| DOX | 0.60 | 1.07 | 1.79 | |
| cis-Pt | 4.68 | 7.87 | 1.68 | |
| 4-hydroxycoumarin | >100 | >100 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, R.; Hou, Z.; Li, J.-T.; Yu, H.-N.; Mou, Y.-H.; Guo, C. Design, Synthesis and Biological Evaluation of Novel 4-Substituted Coumarin Derivatives as Antitumor Agents. Molecules 2018, 23, 2281. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092281
An R, Hou Z, Li J-T, Yu H-N, Mou Y-H, Guo C. Design, Synthesis and Biological Evaluation of Novel 4-Substituted Coumarin Derivatives as Antitumor Agents. Molecules. 2018; 23(9):2281. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092281
Chicago/Turabian StyleAn, Ran, Zhuang Hou, Jian-Teng Li, Hao-Nan Yu, Yan-Hua Mou, and Chun Guo. 2018. "Design, Synthesis and Biological Evaluation of Novel 4-Substituted Coumarin Derivatives as Antitumor Agents" Molecules 23, no. 9: 2281. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092281