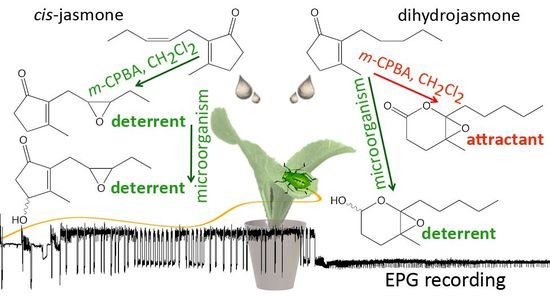
Novel Hydroxy- and Epoxy-cis-Jasmone and Dihydrojasmone Derivatives Affect the Foraging Activity of the Peach Potato Aphid Myzus persicae (Sulzer) (Homoptera: Aphididae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
2.2. Aphid Behavioral Studies
2.2.1. Aphid Settling
2.2.2. Aphid Behavior during Probing in Plant Tissues
3. Material and Methods
3.1. Compounds
3.1.1. Microorganisms
3.1.2. Analysis
3.2. Aphid Behavioral Studies
3.2.1. Cultures of Aphids and Plants
3.2.2. Application of Compounds
3.2.3. Aphid Settling
3.2.4. Aphid Probing Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Staswick, P. Plant hormone conjugation: A signal decision. Plant Signal. Behav. 2009, 4, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.C.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińska-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221–222, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Action of jasmonates in plant stress responses and development—Applied aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Fernandez-Calvo, P.; Schweizer, F.; Goossens, A. Jasmonates: Signal transduction components and their roles in environmental stress responses. Plant Mol. Biol. 2016, 91, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonate signaling in plant stress responses and development—Active and inactive compounds. New Biotechnol. 2016, 33, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Culliney, T. Crop Losses to Arthropods. In Integrated Pest Management; Pimentel, K., Peshin, R., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014; pp. 201–224. [Google Scholar]
- Valenzuela, I.; Hoffman, A.A. Effects of aphid feeding and associated virus injury on grain crops in Australia. Aust. Entomol. 2015, 54, 292–305. [Google Scholar] [CrossRef]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, J.R.; Dachbrodt-Saaydeh, S.; Kudsk, P.; Messean, A. Toward a reduced reliance in conventional pesticides in European agriculture. Plant Dis. 2016, 110, 10–24. [Google Scholar] [CrossRef]
- Pickett, J.A.; Khan, Z.R. Plant volatile-mediated signalling and its application in agriculture: Successes and challenges. New Phytol. 2016, 212, 856–870. [Google Scholar] [CrossRef] [PubMed]
- Slesak, E.; Slesak, M.; Gabrys, B. Effect of methyl jasmonate on hydroxamic acid content, protease activity, and bird cherry-oat aphid Rhopalosiphum padi (L.) probing behavior. J. Chem. Ecol. 2001, 27, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Martin, J.L.; Pickett, J.A.; Pye, B.J.; Smart, L.E.; Wadhams, L.J. cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, Sitobion avenae (Fabricius) (Homoptera: Aphididae). Pest Manag. Sci. 2003, 59, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- El Wakeil, N.E.; Volkmar, C. Effect of jasmonic application on economically insect pests and yield in sprong wheat. Gesunde Pflanzen 2012, 64, 107–116. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Woodcock, C.M.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. cis-Jasmone elicits aphid-induced stress signalling in potatoes. J. Chem. Ecol. 2017, 43, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Birkett, M.A.; Bruce, T.J.; Chamberlain, K.; Gordon-Weeks, R.; Matthes, M.; Napier, J.A.; Smart, L.E.; Woodcock, C.M. Developments in aspects of ecological phytochemistry: The role of cis-jasmone in inducible defence systems in plants. Phytochemistry 2007, 68, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Matthes, M.C.; Chamberlain, K.; Woodcock, C.M.; Mohib, A.; Webster, B.; Smart, L.E.; Birkett, M.A.; Pickett, J.A.; Napier, J.A. cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc. Natl. Acad. Sci. USA 2008, 105, 4553–4558. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Oliveira, J.N.; da Costa, J.G.; Loza-Reyes, E.; Bleicher, E.; Santana, A.E.G.; Caulfield, J.C.; Mayon, P.; Dewhirst, S.Y.; Bruce, T.J.A.; et al. Aphid antixenosis in cotton is activated by the natural plant defence elicitor cis-jasmone. Phytochemistry 2012, 78, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Belsito, D.; Bickers, D.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Miyachi, G.H.Y.; Saurat, J.H.; Sipes, I.G.; et al. A toxicologic and dermatologic assessment of cyclopentanones and cyclopentenones when used as fragrance ingredients. Food Chem. Toxicol. 2012, 50, 517–556. [Google Scholar] [CrossRef] [PubMed]
- Storck, V.G.; Martin-Laurent, F. Towards a better pesticide policy for the European Union. Sci. Total Environ. 2017, 575, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Dancewicz, K.; Białonska, A.; Ciunik, Z.; Gabrys, B.; Wawrzenczyk, C. Synthesis of piperitone-derived halogenated lactones and their effect on aphid probing, feeding, and settling behavior. RSC Adv. 2011, 1, 498–510. [Google Scholar] [CrossRef]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Wawrzeńczyk, C.; Gabrys, B. Piperitone-derived saturated lactones: Synthesis and aphid behavior-modifying activity. J. Agric. Food Chem. 2013, 61, 3364–3372. [Google Scholar] [CrossRef] [PubMed]
- Grudniewska, A.; Kłobucki, M.; Dancewicz, K.; Szczepanik, M.; Gabryś, B.; Wawrzeńczyk, C. Synthesis and antifeedant activity of racemic and optically active hydroxy lactones with the p-menthane system. PLoS ONE 2015, 10, e0131028. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Dancewicz, K.; Hnatejko, M.; Szczepanik, M.; Gabryś, B. Synthesis of β-damascone derivatives with a lactone ring and their feeding deterrent activity against aphids and lesser mealworm. RSC Adv. 2014, 4, 39248–39256. [Google Scholar] [CrossRef]
- Stompor, M.; Dancewicz, K.; Gabrys, B.; Anioł, M. Insect antifeedant potential of xanthohumol, isoxanthohumol, and their derivatives. J. Agric. Food Chem. 2015, 63, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Gabrys, B.; Dancewicz, K.; Gliszczyńska, A.; Kordan, B.; Wawrzeńczyk, C. Systemic deterrence of aphid probing and feeding by β-damascone analogues. J. Pest Sci. 2015, 88, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Boratyński, F.; Dancewicz, K.; Paprocka, M.; Gabryś, B.; Wawrzeńczyk, C. Chemo-enzymatic synthesis of optically active γ- and δ-decalactones and their effect on aphid probing, feeding and settling behavior. PLoS ONE 2016, 11, e0146160. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Gładkowski, W.; Dancewicz, K.; Gabryś, B. Enantioselective microbial hydroxylation as a useful tool in the production of jasmonate derivatives with aphid deterrent activity. Curr. Microbiol. 2015, 71, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Semba, D.; Szczepanik, M.; Dancewicz, K.; Gabryś, B. Alkyl-substituted δ-lactones derived from dihydrojasmone and their stereoselective fungi-mediated conversion: Production of new antifeedant agents. Molecules 2016, 21, 1226. [Google Scholar] [CrossRef] [PubMed]
- Gliszczyńska, A.; Gładkowski, W.; Dancewicz, K.; Gabryś, B.; Szczepanik, M. Transformation of β-damascone to (+)-(S)-4-hydroxy-β-damascone by fungal strains and its evaluation as a potential insecticide against aphid Myzus persicae and lesser mealworm Alphitobius diaperinus Panzer. Catal. Commun. 2016, 80, 39–43. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Tjallingii, W.F.; Garzo, E.; Fereres, A. New structure in cell puncture activities by aphid stylets: A dual-mode EPG study. Entomol. Exp. Appl. 2010, 135, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.-H.; Wang, S.-H.; Liu, T.-X. Jasmonate- and salicylate-induced defenses in wheat affect host preference and probing behavior but not performance of the grain aphid, Sitobion avenae. Insect Sci. 2014, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Horbowicz, M.; Mioduszewska, H.; Koczkodaj, D.; Saniewski, M. The effect of cis-jasmone, jasmoic acid and methyl jasmonate on accumulation of anthocyanins and proanthocyanidins in seedlings of coomon buckwheat (Fagopyrum esculentum Moench). Acta Soc. Bot. Pol. 2009, 4, 271–277. [Google Scholar]
- Fereres, A. Barrier crops as a cultural control measure of non-persistently transmitted aphid-borne viruses. Virus Res. 2000, 71, 221–231. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, J.; Bhatnagar, S.C.; Griffiths, D.C.; Pickett, J.A.; Woodcock, C.M. Activity of qassinoids as antifeedants against aphids. J. Chem. Ecol. 1989, 15, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Hardie, J.; Holyoak, M.; Taylor, N.J.; Griffiths, D.C. The combination of electronic monitoring and video-assisted observations of plant penetration by aphids and behavioural effects of polygodial. Entomol. Exp. Appl. 1992, 62, 233–239. [Google Scholar] [CrossRef]
- Will, T.; Tjallingii, W.F.; Thonnessen, A.; van Bel, A.J. Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. USA 2007, 104, 10536–10541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippi, J.; Schliephake, E.; Jurgens, H.U.; Jansen, G.; Ordon, F. Feeding behavior of aphids on narrow-leafed lupin (Lupinus angustifolius) genotypes varying in the content of quinolizidine alkaloids. Entomol. Exp. Appl. 2015, 156, 37–51. [Google Scholar] [CrossRef]
- Dancewicz, K.; Sznajder, K.; Załuski, D.; Kordan, B.; Gabryś, B. Behavioral sensitivity of Myzus persicae to volatile isporenoids in plant tissues. Entomol. Exp. Appl. 2016, 160, 229–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Francis, F.; Chen, J. Watery saliva secreted by the grain aphid Sitobion avenae stimulates aphid resistance in wheat. J. Agric. Food Chem. 2017, 65, 8798–8805. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Tjallingii, W.F.; Hardie, J. Host plant selection and feeding. In Aphids as Crop Pests; van Emden, H., Harrington, R., Eds.; CAB International: Wallingford, UK, 2007; pp. 87–114. [Google Scholar]
- Tjallingii, W.F. Sieve element acceptance by aphids. Eur. J. Entomol. 1994, 91, 47–52. [Google Scholar]
- Mayoral, A.M.; Tjallingii, W.F.; Castanera, P. Probing behavior of Diuraphis noxia on five cereal species with different hydroxyamic acid levels. Entomol. Exp. Appl. 1996, 78, 341–348. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available currently. However, they may be provided upon request from the authors. |





| EPG Parameter/Compound | C | 1 | 3 | 5 | 6 |
|---|---|---|---|---|---|
| General Aspects of Aphid Probing Behavior | |||||
| n = 17 | n = 16 | n = 12 | n = 14 | n = 22 | |
| Total duration of np a (h) | 0.6 ± 0.3 | 3.1 ± 0.6 * | 2.8 ± 0.7 * | 1.5 ± 0.6 * | 1.2 ± 0.3 *,† |
| Total duration of pathway (h) | 2.9 ± 0.5 | 2.7 ± 0.3 | 3.6 ± 0.6 | 3.0 ± 0.4 | 3.2 ± 0.4 |
| Total duration of phloem phase (h) | 4.5 ± 0.6 | 2.2 ± 0.5 * | 1.7 ± 0.6 * | 3.5 ± 0.6 | 3.5 ± 0.5 |
| Phloem phase index b | 0.6 ± 0.1 | 0.4 ± 0.1 * | 0.3 ± 0.1 * | 0.5 ± 0.1 | 0.5 ± 0.1 † |
| Number of probes | 6.4 ± 2.0 | 21.4 ± 3.0 * | 34.3 ± 7.0 * | 19.9 ± 3.9 * | 22.0 ± 4.1 * |
| Mean duration of a probe (min) | 70.8 ± 13.9 | 13.2 ± 2.4 * | 9.1 ± 1.7 * | 19.6 ± 3.3 * | 18.4 ± 2.8 * |
| Mean duration of np intervals c | 5.8 ± 1.5 | 7.2 ± 1.8 | 4.6 ± 1.4 *,† | 4.7 ± 2.0 | 3.4 ± 0.5 † |
| Activities in Non-Phloem Tissues before the First Phloem Phase | |||||
| n = 17 | n = 16 | n = 12 | n = 14 | n = 22 | |
| Number of probes | 2.1 ± 1.0 | 8.8 ± 2.6 * | 20.1 ± 5.0 * | 4.7 ± 0.8 * | 10.5 ± 2.5 * |
| Duration of first probe (min) | 249.1 ± 54.5 | 10.6 ± 5.1 * | 8.1 ± 4.0 * | 29.9 ± 16.5 * | 10.3 ± 4.9 * |
| Time from first probe to first phloem phase(h) | 2.0 ± 0.5 | 3.5 ± 0.7 | 4.0 ± 0.9 | 1.6 ± 0.5 | 2.1 ± 0.4 |
| Time from first probe to first sustained sap ingestion phase E2 > 10 min (h) | 2.8 ± 0.6 | 4.6 ± 0.6 * | 4.8 ± 0.9 | 3.1 ± 0.7 | 2.5 ± 0.5 † |
| Total duration of np (min) c | 8.5 ± 3.6 | 107.5 ± 40.6 * | 101.4 ± 46.3 * | 36.5 ± 27.7 | 26.3 ± 8.5 * |
| Mean duration of np intervals (min)c | 4.0 ± 1.6 | 11.0 ± 3.6 | 4.8 ± 1.7 *,† | 6.6 ± 4.9 | 2.5 ± 0.5 |
| Activities in Sieve Elements B | |||||
| Duration of first phloem phase (min) | 160.2 ± 47.6 | 60.8 ± 31.9 | 57.8 ± 44.7 | 100.7 ± 41.4 | 149.8 ± 33.0 † |
| n = 16 | n = 12 | n = 8 | n = 13 | n = 21 | |
| Duration of first sap ingestion phase (min) | 159.7 ± 47.6 | 63.3 ± 31.4 | 92.2 ± 51.6 | 110.5 ± 43.7 | 156.5 ± 33.7 † |
| n = 16 | n = 12 | n = 8 | n = 12 | n = 20 | |
| Mean duration of sap ingestion (min) | 175.8 ± 44.9 | 81.0 ± 30.0 | 101.0 ± 50.3 | 107.9 ± 39.7 | 162.7 ± 31.7 |
| n = 16 | n = 12 | n = 8 | n = 12 | n = 20 | |
| Phloem salivation index d | 0.1 ± 0.0 | 0.04 ± 0.01 | 0.1 ± 0.0 * | 0.1 ± 0.1 | 0.1 ± 0.1 |
| n = 16 | n = 12 | n = 8 | n = 13 | n = 21 | |
| EPG Parameter/Compound | C | 2 | 4 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|
| General aspects of aphid probing behaviour | |||||||
| n = 17 | n = 15 | n = 14 | n = 14 | n = 15 | n = 11 | n = 18 | |
| Total duration of np a (h) | 0.6 ± 0.3 | 2.6 ± 0.8 * | 1.5 ± 0.4 * | 1.6 ± 0.4 * | 1.6 ± 0.4 * | 2.0 ± 0.8 * | 1.3 ± 0.5 * |
| Total duration of pathway (h) | 2.9 ± 0.5 | 1.8 ± 0.4 | 3.9 ± 0.7 † | 2.8 ± 0.5 | 3.4 ± 0.4 † | 3.5 ± 0.7 | 2.8 ± 0.5 |
| Total duration of phloem phase (h) | 4.5 ± 0.6 | 3.6 ± 0.7 | 2.7 ± 0.8 | 3.7 ± 0.6 | 3.0 ± 0.6 | 2.4 ± 0.9 | 3.9 ± 0.7 |
| Phloem phase index b | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 |
| Number of probes | 6.4 ± 2.0 | 12.3 ± 2.8 * | 23.4 ± 4.2 *,† | 16.5 ± 3.3 * | 28.0 ± 6.2 *,† | 26.0 ± 5.2 *,† | 19.1 ± 3.6 * |
| Mean duration of a probe (min) | 70.8 ± 13.9 | 26.5 ± 5.7 * | 16.7 ± 3.1 *,† | 23.3 ± 4.4 * | 13.7 ± 2.3 *,† | 13.8 ± 2.8 *,† | 20.9 ± 3.9 *,† |
| Mean duration of np intervals c | 5.8 ± 1.5 | 13.2 ± 4.7 | 3.9 ± 0.8 | 4.6 ± 1.4 | 3.1 ± 0.8 *,† | 3.7 ± 1.6 *,† | 3.7 ± 1.0 |
| Activities in non-phloem tissues before 1st phloem phase | |||||||
| n = 17 | n = 15 | n = 14 | n = 14 | n = 15 | n = 11 | n = 18 | |
| Number of probes | 2.1 ± 1.0 | 7.1 ± 2.5 * | 14.7 ± 3.4 * | 7.2 ± 1.6 * | 6.5 ± 1.5 * | 14.0 ± 4.6 * | 10.3 ± 2.6 * |
| Duration of first probe (min) | 249.1 ± 54.5 | 37.4 ± 25.9 * | 34.6 ± 30.0 * | 8.3 ± 5.7 * | 16.9 ± 8.2 * | 5.6 ± 2.7 * | 66.7 ± 36.1 * |
| Time from first probe to first phloem phase(h) | 2.0 ± 0.5 | 2.9 ± 0.8 | 3.6 ± 0.8 | 1.6 ± 0.3 | 1.4 ± 0.2 | 3.2 ± 1.0 | 2.6 ± 0.6 |
| Time from first probe to first sustained sap ingestion phase E2> 10 min (h) | 2.8 ± 0.6 | 3.3 ± 0.8 | 3.8 ± 1.0 | 2.7 ± 0.6 | 2.4 ± 0.5 | 5.0 ± 1.0 | 3.0 ± 0.7 |
| Total duration of np (min) c | 8.5 ± 3.6 | 119.1 ± 49.6 * | 69.2 ± 27.7 * | 17.5 ± 4.9 * | 13.2 ± 6.0 | 56.0 ± 36.4 * | 44.2 ± 24.0 * |
| Mean duration of np intervals (min) c | 4.0 ± 1.6 | 16.7 ± 6.8 | 4.8 ± 1.2 | 2.4 ± 0.4 | 2.0 ± 0.7 *,† | 4.0 ± 2.6 *,† | 4.3 ± 1.8 *,† |
| Activities in sieve elements A | |||||||
| Duration of first phloem phase (min) | 160.2 ± 47.6 | 124.4 ± 49.5 | 131.0 ± 53.0 | 160.5 ± 42.8 | 38.7 ± 17.1 | 80.3 ± 53.1 | 211.6 ± 50.0 |
| n = 16 | n = 11 | n = 11 | n = 14 | n = 15 | n = 8 | n = 16 | |
| Duration of first sap ingestion phase (min) | 159.7 ± 47.6 | 161.2 ± 53.3 | 132.8 ± 58.5 | 170.6 ± 44.7 | 60.0 ± 24.8 | 108.4 ± 58.2 | 211.4 ± 49.8 |
| n = 16 | n = 11 | n = 10 | n = 13 | n = 15 | n = 7 | n = 16 | |
| Mean duration of sap ingestion (min) | 175.8 ± 44.9 | 167.6 ± 45.0 | 151.5 ± 55.7 | 164.3 ± 45.3 | 68.2 ± 22.3 * | 135.8 ± 56.4 | 224.7 ± 47.2 |
| n = 16 | n = 11 | n = 10 | n = 13 | n = 15 | n = 7 | n = 16 | |
| Phloem salivation index d | 0.1 ± 0.0 | 0.01 ± 0.00 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.0 † | 0.2 ± 0.1 | 0.03 ± 0.01 |
| n = 16 | n = 11 | n = 11 | n = 14 | n = 15 | n = 8 | n = 17 | |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paprocka, M.; Gliszczyńska, A.; Dancewicz, K.; Gabryś, B. Novel Hydroxy- and Epoxy-cis-Jasmone and Dihydrojasmone Derivatives Affect the Foraging Activity of the Peach Potato Aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Molecules 2018, 23, 2362. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092362
Paprocka M, Gliszczyńska A, Dancewicz K, Gabryś B. Novel Hydroxy- and Epoxy-cis-Jasmone and Dihydrojasmone Derivatives Affect the Foraging Activity of the Peach Potato Aphid Myzus persicae (Sulzer) (Homoptera: Aphididae). Molecules. 2018; 23(9):2362. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092362
Chicago/Turabian StylePaprocka, Marlena, Anna Gliszczyńska, Katarzyna Dancewicz, and Beata Gabryś. 2018. "Novel Hydroxy- and Epoxy-cis-Jasmone and Dihydrojasmone Derivatives Affect the Foraging Activity of the Peach Potato Aphid Myzus persicae (Sulzer) (Homoptera: Aphididae)" Molecules 23, no. 9: 2362. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23092362