Design, Synthesis, and Evaluation of Alkyl-Quinoxalin-2(1H)-One Derivatives as Anti-Quorum Sensing Molecules, Inhibiting Biofilm Formation in Aeromonas caviae Sch3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of Quorum Sensing Inhibitors and Structural Development
2.2. Synthesis
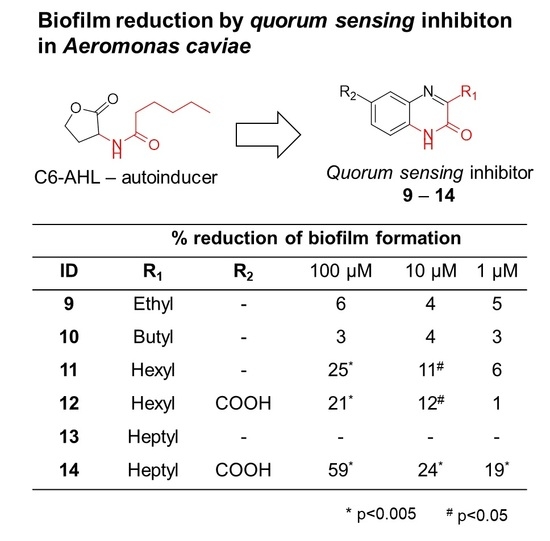
2.3. Inhibition of Biofilm Formation by Anti-Quorum Sensing Compounds
2.4. Evaluation of Quorum Sensing Inhibition Through Reduction of Violacein Production in Chromobacterium Violaceum CV026
3. Materials and Methods
3.1. Chemistry
3.1.1. General Method for the Synthesis of Compounds 9–14, Described at the Example of 3-Ethylquinoxalin-2(1H)-one (8)
3.1.2. Analytics
3-Ethylquinoxalin-2(1H)-one (9)

3-Butylquinoxalin-2(1H)-one (10)

3-Hexylquinoxalin-2(1H)-one (11)

3-Hexylquinoxalin-2(1H)-one-6-carboxylic acid (12)

3-Heptylquinoxalin-2(1H)-one (13)

3-Heptylquinoxalin-2(1H)-one-6-carboxylic acid (14)

3.2. Bacteriology
3.2.1. Bacterial Strain Aeromonas caviae Sch3
3.2.2. Culture Conditions Aeromonas caviae Sch3
3.2.3. Evaluation of Biofilm Reduction
3.2.4. Culture Conditions Chromobacterium violaceum CV026
3.2.5. Evaluation of Compounds as Quorum Sensing Inhibitors in Chromobacterium violaceum CV026
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rampioni, G.; Leoni, L.; Williams, P. The art of antibacterial warfare: Deception through interference with quorum sensing-mediated communication. Bioorg. Chem. 2014, 55, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zambelloni, R.; Marquez, R.; Roe, A.J. Development of antivirulence compounds: A biochemical review. Chem. Biol. Drug Des. 2015, 85, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Alasil, S.M.; Omar, R.; Ismail, S.; Yusof, M.Y. Inhibition of Quorum Sensing-Controlled Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa by Culture Extract from Novel Bacterial Species of Paenibacillus Using a Rat Model of Chronic Lung Infection. Int. J. Bacteriol. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lazdunski, A.M.; Ventre, I.; Sturgis, J.N. Regulatory circuits and communication in gram-negative bacteria. Nat. Rev. Microbiol. 2004, 2, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Rasch, M.; Kastbjerg, V.G.; Bruhn, J.B.; Dalsgaard, I.; Givskov, M.; Gram, L. Quorum sensing signals are produced by Aeromonas salmonicida and quorum sensing inhibitors can reduce production of a potential virulence factor. Dis. Aquat. Organ. 2007, 78, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survivalmechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Talagrand-Reboul, E.; Jumas-Bilak, E.; Lamy, B. The social life of Aeromonas through biofilm and quorum sensing systems. Front. Microbiol. 2017, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Val, D.L.; Hanzelka, B.L.; Cronan, J.E.; Greenberg, E.P. Acyl homoserine-lactone quorum-sensing signal generation. Proc. Natl. Acad. Sci. USA 1999, 96, 4360–4365. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.; Karlyshev, A.V.; Fish, L.; Durant, E.L.; Winson, M.K.; Chhabra, S.R.; Williams, P.; Macintyre, S.; Stewart, G.S. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: Identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J. Bacteriol. 1997, 179, 5271–5281. [Google Scholar] [CrossRef] [PubMed]
- LaSarre, B.; Federle, M.J. Exploiting Quorum Sensing To Confuse Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.M.; Queneau, Y.; Soulère, L.; Von Bodman, S.; Doutheau, A. Mechanisms and synthetic modulators of AHL-dependent gene regulation. Chem. Rev. 2011, 111, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.J.; De Vlieg, J.; Wagener, M.; Ritschel, T. Pharmacophore fingerprint-based approach to binding site subpocket similarity and its application to bioisostere replacement. J. Chem. Inf. Model. 2012, 52, 2031–2043. [Google Scholar] [CrossRef] [PubMed]
- Moser, D.; Wittmann, S.K.; Kramer, J.; Blöcher, R.; Achenbach, J.; Pogoryelov, D.; Proschak, E. PENG: A Neural Gas-Based Approach for Pharmacophore Elucidation. Method Design, Validation, and Virtual Screening for Novel Ligands of LTA4H. J. Chem. Inf. Model. 2015, 55, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yu, Y.; Hua, Y.; Feng, F.; Tong, Y.; Yang, X.; Xiao, J.; Song, H. Design, synthesis and biological evaluation of N-sulfonyl homoserine lactone derivatives as inhibitors of quorum sensing in Chromobacterium violaceum. Molecules 2013, 18, 3266–3278. [Google Scholar] [CrossRef] [PubMed]
- Swem, L.R.; Swem, D.L.; O’Loughlin, C.T.; Gatmaitan, R.; Zhao, B.; Ulrich, S.M.; Bassler, B.L. A Quorum-Sensing Antagonist Targets Both Membrane-Bound and Cytoplasmic Receptors and Controls Bacterial Pathogenicity. Mol. Cell 2009, 35, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Mcclean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; John, H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acyl homoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.J.A.; Cheng, X.; List, B. Organocatalytic asymmetric transferhydrogenation of beta-nitroacrylates: Accessing beta2-amino acids. J. Am. Chem. Soc. 2008, 130, 13862–13863. [Google Scholar] [CrossRef] [PubMed]
- Koz’minykh, V.O.; Goncharov, V.I. Simple procedure for preparation of quinoxalin-2(1H)-one 3-[oxo(cyclo)alkyl(idene)] derivatives. Russ. J. Org. Chem. 2006, 42, 1715–1718. [Google Scholar] [CrossRef]
- Molle, G.; Bauer, P. The Barbier Synthesis: A One-Step Grignard Reaction? J. Am. Chem. Soc. 1982, 104, 3481–3487. [Google Scholar] [CrossRef]
- Beatriz Angeles-Morales, E. Evaluation of Morphological Changes of Aeromonas caviae Sch3 Biofilm Formation under Optimal Conditions. Adv. Microbiol. 2012, 2, 552–560. [Google Scholar] [CrossRef]
- Galloway, W.R.J.; Hodgkinson, J.T.; Bowden, S.D.; Welch, M.; Spring, D.R. Quorum sensing in Gram-negative bacteria: Small-molecule modulation of AHL and AI-2 quorum sensing pathways. Chem. Rev. 2011, 111, 28–67. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 9, 10, 11, 12, 13 and 14 are available from the corresponding author Dr. Alicia Reyes-Arellano. |




 | % Reduction of Biofilm Formation | ||||
| ID | R1 | R2 | 100 µM | 10 µM | 1 µM |
| 9 | Ethyl | - | 6 | 4 | 5 |
| 10 | Butyl | - | 3 | 4 | 3 |
| 11 | Hexyl | - | 25 * | 11 # | 6 |
| 12 | Hexyl | COOH | 21 * | 12 # | 1 |
| 13 | Heptyl | - | - | - | - |
| 14 | Heptyl | COOH | 59 * | 24 * | 19 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blöcher, R.; Rodarte Ramírez, A.; Castro-Escarpulli, G.; Curiel-Quesada, E.; Reyes-Arellano, A. Design, Synthesis, and Evaluation of Alkyl-Quinoxalin-2(1H)-One Derivatives as Anti-Quorum Sensing Molecules, Inhibiting Biofilm Formation in Aeromonas caviae Sch3. Molecules 2018, 23, 3075. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123075
Blöcher R, Rodarte Ramírez A, Castro-Escarpulli G, Curiel-Quesada E, Reyes-Arellano A. Design, Synthesis, and Evaluation of Alkyl-Quinoxalin-2(1H)-One Derivatives as Anti-Quorum Sensing Molecules, Inhibiting Biofilm Formation in Aeromonas caviae Sch3. Molecules. 2018; 23(12):3075. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123075
Chicago/Turabian StyleBlöcher, René, Ariel Rodarte Ramírez, Graciela Castro-Escarpulli, Everardo Curiel-Quesada, and Alicia Reyes-Arellano. 2018. "Design, Synthesis, and Evaluation of Alkyl-Quinoxalin-2(1H)-One Derivatives as Anti-Quorum Sensing Molecules, Inhibiting Biofilm Formation in Aeromonas caviae Sch3" Molecules 23, no. 12: 3075. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123075