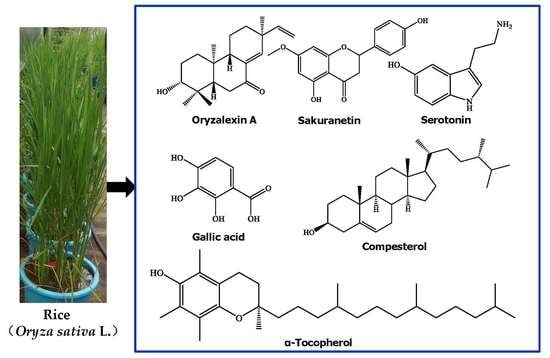
Rice Secondary Metabolites: Structures, Roles, Biosynthesis, and Metabolic Regulation
Abstract
:1. Introduction
2. Structural Diversity and Roles of Rice Secondary Metabolites
2.1. Phenolic Acids and Their Biological Functions
2.2. Flavonoids and Their Biological Functions
2.3. Terpenoids and Their Biological Functions
2.3.1. Monoterpenoids and Their Biological Functions
2.3.2. Sesquiterpenoids and Their Biological Functions
2.3.3. Diterpenoids and Their Biological Functions
2.3.4. Triterpenoids and Their Biological Functions
2.4. Steroids and Their Biological Functions
2.5. Alkaloids and Their Biological Functions
2.6. Other Metabolites
3. Biosynthetic Pathways of Rice Secondary Metabolites
3.1. Biosynthesis of Flavonoids
3.2. Biosynthesis of Terpenoids
3.3. Biosynthesis of Tocotrienol and Tocopherol
3.4. Biosynthesis of Alkaloids
4. Metabolic Regulation of Secondary Meatobolites
4.1. Metabolic Regulation by Abiotic Stresses
4.1.1. Metabolic Regulation by Phytohormones
4.1.2. Metabolic Regulation by Oligosaccharides
4.1.3. Metabolic Regulation by Cerebrosides
4.1.4. Metabolic Regulation by Cholic Acid
4.1.5. Metabolic Regulation by Heavy Metal Ions
4.1.6. Metabolic Regulation by Ultraviolet Irradiation
4.1.7. Metabolic Regulation by Other Abiotic Stresses
4.2. Metabolic Regulation by Biotic Stresses
4.2.1. Metabolic Regulation by Bacteria
4.2.2. Metabolic Regulation by Fungi
4.2.3. Metabolic Regulation by Insect Pests
4.2.4. Metabolic Regulation by Nematodes
4.2.5. Metabolic Regulation by Viruses
4.2.6. Metabolic Regulation by Other Plants
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Z.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Tech. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Deng, G.-F.; Xu, X.-R.; Zhang, Y.; Li, D.; Gan, R.-Y.; Li, H.-B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Izawa, T.; Shimamoto, K. Becoming a model plant: The importance of rice to plant science. Trends Plant Sci. 1996, 1, 95–99. [Google Scholar] [CrossRef]
- Friedman, M. Rice brans, rice bran oils, and rice hulls: Composition, food and industrial uses, and bioactivies in humans, animals, and cells. J. Agric. Food Chem. 2013, 61, 10626–10641. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-Lopez, A.; Giampieri, F.; Battino, M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.; Goslinski, M.; Wojtowicz, E.; Przygonski, K. Antioxidant properties and phenolic compounds of vitamin C-rich juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Stout, M.; Qian, Q.; Chen, F. Genetic, molecular and genomic basis of rice defense against insects. Crit. Rev. Plant Sci. 2012, 31, 74–91. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Peters, R.J. The role of momilactones in rice allelopathy. J. Chem. Ecol. 2013, 39, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H. Biosynthesis of phytoalexins and regulatory mechanisms of it in rice. Biosci. Biotechnol. Biochem. 2013, 77, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, antocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, M.-H.; Lee, S.-W. Phenolic phytoalexins in rice: Biological functions and biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef] [PubMed]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Routray, W.; Rayaguru, K. 2-Acetyl-1-pyrroline: A key aroma component of aromatic rice and other food products. Food Rev. Int. 2018, 34, 539–565. [Google Scholar] [CrossRef]
- Goff, S.; Stephen, A.; Riche, D.; Lan, T.-H.; Presting, G.; Wang, R.; Dunn, M.; Glazebrook, J.; Sessioins, A.; Oeller, P.; et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 2002, 296, 92–101. [Google Scholar] [PubMed]
- Yu, J.; Ju, S.; Wang, J.; Wong, G.K.-S.; Li, S.; Liu, B.; Deng, Y.; Dai, L.; Zhou, Y.; Zhang, X.; et al. A draft sequence of the rice genome (Oryza sativa L. spp. indica). Science 2002, 296, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Shimizu, T.; Okada, K. Transcriptional regulation of the biosynthesis of phytoalexin: A lesson from specialized metabolites in rice. Plant Biotechnol. 2014, 31, 377–388. [Google Scholar] [CrossRef] [Green Version]
- Kusano, M.; Yang, Z.; Okazaki, Y.; Nakabayashi, R.; Fukushima, A.; Saito, K. Using metabolomics approaches to explore chemical diversity in rice. Mol. Plant 2015, 8, 58–67. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2013, 152, 46–55. [Google Scholar] [CrossRef]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, J.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in Southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, Y.; Bao, J.; Beta, T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015, 172, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Zaupa, M.; Calani, L.; Rio, D.D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly)phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Liu, Q.; Li, P.; Pei, Y.; Tao, T.; Wang, Y.; Yan, W.; Yang, G.; Shao, X. Distribution and quantitative analysis of phenolic compounds in fractions of Japonica and Indica rice. Food Chem. 2019, 274, 384–391. [Google Scholar] [CrossRef]
- Jun, H.-I.; Shin, J.-W.; Song, G.-S.; Kim, Y.-S. Isolation and identification of phenolic antioxidants in black rice bran. J. Food Sci. 2015, 89, C262–C268. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J.S. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Seal, A.N.; Pratley, J.E.; Haig, T.; An, M. Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. J. Chem. Ecol. 2004, 30, 1647–1662. [Google Scholar] [CrossRef]
- Olofsdotter, M.; Rebulanan, M.; Madrid, A.; Wang, D.; Navarez, D.; Olk, D.C. Why phenolic acids are unlikely primary allelochemicals in rice. J. Chem. Ecol. 2002, 28, 229–242. [Google Scholar] [CrossRef]
- Seal, A.N.; Haig, T.; Pratley, J.E. Evaluation of putative allelochemicals in rice root exudates for their role in the suppression of arrowhead root growth. J. Chem. Ecol. 2004, 30, 1663–1678. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J.; Zhang, J.; Peng, J.; Liu, T.; Xin, Z. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015, 171, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Nakanishi, T.; Shimoda, H.; Nakamura, S.; Tsuruma, K.; Shimazawa, M.; Matsuda, H.; Yoshikawa, M.; Hara, H. Purple rice extract and its constituents suppress endoplasmic reticulum stress-induced retinal damage in vitro and in vivo. Life Sci. 2013, 92, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Hu, X.; McClements, D.J.; Luo, S.; Liu, C. Hydrothermal stability of phenolic extracts of brown rice. Food Chem. 2019, 271, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nakabayashi, R.; Okazaki, Y.; Mori, T.; Takamatsu, S.; Kitanaka, S.; Kikuchi, J.; Saito, K. Toward better annotation in plant metabolomics: Isolation and structure elucidation of 36 specialized metabolites from Oryza sativa (rice) by using MS/MS and NMR analyses. Metabolomics 2014, 10, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Besson, E.; Dellamonica, G.; Chopin, J.; Markham, K.R.; Kim, M.; Koh, H.-S.; Fukami, H. C-Glycosylflavones from Oryza sativa. Phytochemistry 1985, 24, 1061–1064. [Google Scholar] [CrossRef]
- Grayer, R.J.; Harborne, J.B.; Kimmins, F.M.; Stevenson, P.C.; Wijayagunasekera, H.N.P. Phenolics in rice phloem sap as sucking deterrents to the brown planthopper, Nilaparvata lugens. Acta Hort. 1994, 381, 691–694. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kimmins, F.M.; Grayer, R.J.; Raveendranath, S. Schaftosides from rice phloem as feeding inhibitors and resistance factors to brown planthoppers, Nilaparvata lungens. Entomol. Exp. Appl. 1996, 80, 246–249. [Google Scholar] [CrossRef]
- Mohanlal, S.; Maney, S.K.; Santhoshkumar, T.R.; Jayalekshmy, A. Tricin 4′-O-(erythro-β-guaiacylglyceryl) ether and tricin 4′-O-(threo-β-guaiacylglyceryl) ether isolated from Njavara (Oryza sativa L. var. Njavara), induce apoptosis in multiple tumor cells by mitochondrial pathway. J. Nat. Med. 2013, 67, 528–533. [Google Scholar] [CrossRef]
- Cho, J.-G.; Song, N.-Y.; Nam, T.-G.; Shrestha, S.; Park, H.-J.; Lyu, H.-N.; Kim, D.-O.; Lee, G.; Woo, Y.-M.; Jeong, T.-S.; et al. Flavonoids from the grains of C1/R-S transgenic rice, the transgenic Oryza sativa spp. japonica, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 10354–10359. [Google Scholar]
- Kong, C.H.; Li, H.B.; Hu, F.; Xu, X.H.; Wang, P. Allelochemicals released by rice roots and residues in soil. Plant Soil 2006, 288, 47–56. [Google Scholar] [CrossRef]
- Kong, C.H.; Zhao, H.; Xu, X.H.; Wang, P.; Gu, Y. Activity and allelopathy of soil of flavone O-glycosides from rice. J. Agric. Food Chem. 2007, 55, 6007–6012. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lin, F.; Hasegawa, M.; Okada, K.; Nojiri, H.; Yamane, H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J. Biol. Chem. 2012, 287, 19315–19325. [Google Scholar] [CrossRef] [PubMed]
- Kodama, O.; Miyakawa, J.; Akatsuka, T.; Kiyosawa, S. Sakuranetin, a flavanone phytoalexin from ultraviolet-irradiated rice leaves. Phytochemistry 1992, 31, 3807–3809. [Google Scholar] [CrossRef]
- Katsumata, S.; Hmamna, K.; Horie, K.; Toshima, H.; Hasegawa, M. Identification of sternbin and naringenin as detoxified metabolites from the rice flavanone phytoalexin sakuranetin by Pyricularia oryzae. Chem. Biodivers. 2017, 14, e1600240. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, S.; Toshima, H.; Hasegawa, M. Xylosylated detoxification of the rice flavonoid phytoalexin sakuranetin by the rice sheath blight fungus Rhizoctonia solani. Molecules 2018, 23, 276. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Abe, D.; Sekiya, K. Sakuranetin induces adipopenesis of 3T3-L1 cells through enhanced expression of PPARγ2. Biochem. Biophys. Res. Commun. 2008, 372, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.-S.; Song, K.-S.; Seong, Y.H.; Bai, K.H. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kinoshita, H.; Okuno, Y. Antimutagenic activity of sakuranetin from Prunus jamasakura. J. Food Sci. 2003, 68, 52–56. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, Y.; Wu, D.; Zhang, H.; Wu, J.; Chen, J.; Ding, J.; Hu, L.; Jiang, H.; Shen, X. Three flavonoids targeting the β-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter pylori: Crystal structure characterization with enzymatic inhibition assay. Protein Sci. 2008, 17, 1971–1978. [Google Scholar] [CrossRef]
- Grecco, S.S.; Reimao, J.Q.; Tempone, A.G.; Sartorelli, P.; Cunha, R.L.; Romoff, P.; Ferreira, M.J.P.; Favero, O.A.; Lago, J.H.G. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC (Asteraceae). Exp. Parasitol. 2012, 130, 141–145. [Google Scholar] [CrossRef]
- Drira, R.; Sakamoto, K. Sakuranetin induces melanogenesis in B16BL6 melanoma cells through inhibition of ERK and PI3K/AKT signaling pathways. Phytother. Res. 2016, 30, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhu, H.; Zhang, Z.; Yang, S.; Li, H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Yang, Z.; Nakabayashi, R.; Mori, T.; Takamatsu, S.; Kitanaka, S.; Saito, K. Metabolome analysis of Oryza sativa (rice) using liquid chromatography-mass spectrometry for characterizing organ specificity of flavonoids with anti-inflammatory and anti-oxidant activity. Chem. Pharm. Bull. 2016, 64, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Xu, X.; Zhou, B.; Hu, F.; Zhang, C.; Zhang, M. Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens. Phytochemistry 2004, 65, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Liang, W.; Xu, X.; Hu, F.; Wang, P.; Jiang, Y. Release and activity of allelochemicals from allelopathic rice seedlings. J. Agric. Food Chem. 2004, 52, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, M.J.; Mohanlal, S.; Suresh, C.H.; Jayalekshmy, A. DPPH radical scavenging activity of tricin and its conjugates isolated from “Njavara” rice bran: A density functional theory study. J. Agric. Food Chem. 2012, 60, 3693–3699. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.N.; Samanidou, V.F.; Biliaderis, C.G.; Papadoyannis, I.N. Simultaneous determination of phenolic acids and flavonoids in rice using solid-phase extraction and RP-HPLC with photodiode array detection. J. Sep. Sci. 2012, 35, 1603–1611. [Google Scholar] [CrossRef]
- Park, H.L.; Yoo, Y.; Hahn, T.-R.; Bhoo, S.-H.; Lee, S.-W.; Cho, M.-H. Antimicrobial activity of UV-induced phenylamides from rice leaves. Molecules 2014, 19, 18139–18151. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and pH degradation kinetics of anthocyanins in natural food colorant prepared from black rice bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef]
- Tamura, S.; Yan, K.; Shimoda, H.; Murakami, N. Anthocyanins from Oryza sativa L. subsp. indica. Biochem. Syst. Ecol. 2010, 38, 438–440. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Kazuya, A.; Gomi, K. Rice terpene synthase 24 (PsTPS24) encodes a jamonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.W.; Chung, M.-S.; Kang, M.; Chung, B.Y.; Lee, S. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma 2016, 253, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Chumpolsri, W.; Wijit, N.; Boontakham, P.; Nimmanpipug, P.; Sookwong, P.; Luangkamin, S.; Wongpornchai, S. Variation of terpenoid flavor odorants in bran of some black and white rice varieties analyzed by GC×GC-MS. J. Food Nutr. Res. 2015, 3, 114–120. [Google Scholar] [CrossRef]
- Obara, N.; Hasegawa, M.; Kodama, O. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci. Biotechnol. Biochem. 2002, 66, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Kiryu, M.; Hamanaka, M.; Yoshitomi, K.; Mochizuki, S.; Akimitsu, K.; Gomi, K. Rice terpene synthase 18 (OsTPS18) encodes a sesquitperpene synthase that produces and antibacterial (E)-nerolidol against a bacterial pathogen of rice. J. Gen. Plant Pathol. 2018, 84, 221–229. [Google Scholar] [CrossRef]
- Changan, S.S.; Ali, K.; Kumar, V.; Garg, N.K.; Tyagi, A. Abscisic acid biosynthesis under water stress: Anomalous behavior of the 9-cis-epoxycarotenoid dioxygenase1 (NCED1) gene in rice. Biol. Plantarum 2018, 62, 663–670. [Google Scholar] [CrossRef]
- Kurogochi, S.; Murofushi, N.; Ota, Y.; Takahashi, N. Identification of gibberellins in the rice plant and quantitative changes of gibberellin A19 throught its life cycle. Planta 1979, 146, 185–191. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Lancake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Horie, K.; Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Koga, J.; Toshima, H.; Hasegawa, M. Identification of UV-induced diterpenes including a new diterpene phytoalexin, phytocassane F, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food Chem. 2015, 63, 4050–4059. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, T.; Kadama, O.; Kato, H.; Kono, Y.; Takeuchi, S. 3-Hydroxy-7-oxo-sandaraco-pimaradiene (oryzalexin A), a new phytoalexin isolated from rice blast leaves. Agric. Biol. Chem. 1983, 47, 445–447. [Google Scholar] [CrossRef]
- Kono, Y.; Takeuchi, S.; Kodama, O.; Akatsuka, T. Absolute configuration of oryzalexin A and structures of its related phytoalexins isolated from rice blast leaves infected with Pyricularia oryzae. Agric. Biol. Chem. 1984, 48, 253–255. [Google Scholar] [CrossRef]
- Akatsuka, T.; Kodama, O.; Sekido, H.; Kono, Y.; Takeuchi, S. Novel phytoalexins (oryzlexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part I: Isolation, characterization and biological activities of oryzalexins. Agric. Biol. Chem. 1985, 49, 1689–1694. [Google Scholar]
- Kono, Y.; Takeuchi, S.; Kodama, O.; Sekido, H.; Akatsuka, T. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part II: Structural studies of oryzalexins. Agric. Biol. Chem. 1985, 49, 1695–1701. [Google Scholar] [CrossRef]
- Sekido, H.; Endo, T.; Suga, R.; Kodama, O.; Akatsuka, T.; Kono, Y.; Takeuchi, S. Oryzalexin D (3,7-dihydroxy-(+)-sandaracopimaradiene), a new phytoalexin isolated from blast-infected rice leaves. Nippon Noyaku Gakkaishi 1986, 11, 369–372. [Google Scholar]
- Kato, H.; Kodama, O.; Akatrsuka, T. Oryzalexin E, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1993, 33, 79–81. [Google Scholar] [CrossRef]
- Kato, H.; Kodama, O.; Akatsuka, T. Oryzalexin F, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1994, 36, 299–301. [Google Scholar] [CrossRef]
- Koga, J.; Shimura, M.; Oshima, K.; Ogawa, N.; Yamauchi, T.; Ogasawara, N. Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron 1995, 51, 7907–7918. [Google Scholar] [CrossRef]
- Koga, J.; Ogawa, N.; Yamauchi, T.; Kikuchi, M.; Ogasawara, N.; Shimura, M. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry 1997, 44, 249–253. [Google Scholar] [CrossRef]
- Yajima, A.; Mori, K. Diterpenoid total synthesis, XXXII synthesis and absolute configuration of (-)-phytocassane D, a diterpene phytoalexin isolated from the rice plant, Oryza sativa. Eur. J. Org. Chem. 2000, 2000, 4079–4091. [Google Scholar] [CrossRef]
- Horie, K.; Sakai, K.; Okugi, M.; Toshima, H.; Hasegawa, M. Ultraviolet-induced amides and casbene diterpenoids from rice leaves. Phytochm. Lett. 2016, 15, 57–62. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Toshima, H.; Hasegawa, M. Identification of a novel casbane-type diterpene phytoalexin, ent-10-oxodepressin, from rice leaves. Biosci. Biotechnol. Biochem. 2013, 77, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kono, Y.; Uzawa, J.; Teraoka, T.; Hosokawa, D.; Suzuki, Y.; Sakurai, A.; Teraguchi, M. Structures of oryzalic acid B and three related compounds, a group of novel antibacterial diterpenes, isolated from leaves of a bacterial leaf blight-resistant cultivar of rice. Biosci. Biotechnol. Biochem. 1992, 56, 113–117. [Google Scholar] [CrossRef]
- Kono, Y.; Kojima, A.; Nagai, R.; Watanabe, M.; Onizawa, T.; Teraoka, T.; Watanab, M.; Koshino, H.; Uzawa, J.; Suzuki, Y.; et al. Antibacterial diterpenenes and their fatty acid conjugates from rice leaves. Phytochemistry 2004, 65, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Sakai, Y.; Teraoka, T.; Abe, H.; Kono, Y.; Uzawa, J.; Kobayashi, K.; Suzuki, Y.; Sakurai, A. Novel C19-kaurane type of diterpene (Oryzalide A), a nve antimicrobial compound isolated from healthy leaves of a bacterial leaf blight-resistant cultivar of rice plant. Agric. Biol. Chem. 1990, 54, 1103–1105. [Google Scholar] [CrossRef]
- Kono, Y.; Uzawa, J.; Kobayashi, K.; Suzuki, Y.; Uramoto, M.; Sakurai, A.; Watanabe, M.; Teraoka, T.; Hosokawa, D.; Watanabe, M.; et al. Structures of oryzalides A and B, and oryzalic acid A, a group of novel antimicrobial diterpenes, isolated from healthy leaves of a bacterial leaf blight-resistant cultivar of rice plant. Agric. Biol. Chem. 1991, 55, 803–811. [Google Scholar] [CrossRef]
- Watanabe, M.; Kono, Y.; Esumi, Y.; Teraoka, T.; Hosokawa, D.; Suzuki, Y.; Sakurai, A.; Watanabe, M. Studies on a quantitative analysis of oryzalides and oryzalic acids in rice plants by GC-SIM. Biosci. Biotechnol. Biochem. 1996, 60, 1460–1463. [Google Scholar] [CrossRef]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 39, 3861–3864. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ota, K.; Ino, T. Release of momilactone A and B from rice plants into the rhizosphere and its bioactivities. Allelopathy J. 2008, 22, 321–328. [Google Scholar]
- Kato-Noguchi, H.; Ino, T. Concentration and release level of momilactone B in the seedlings of eight rice cultivars. J. Plant Physiol. 2005, 162, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, J.T.; Kim, S.-H. Evaluation of allelopathic potential and quantification of momilactone A, B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food Chem. 2006, 54, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Tsunakawa, M.; Ohba, A.; Sasaki, N.; Kabuto, C.; Kato, T.; Kitahara, Y.; Takahashi, N. Momilactone C, a minor constituent of growth inhibitors in rice husk. Chem. Lett. 1976, 5, 1157–1158. [Google Scholar] [CrossRef]
- Cho, J.-G.; Cha, B.-J.; Lee, S.-M.; Shrestha, S.; Jeong, R.-H.; Lee, D.S.; Kim, Y.-C.; Lee, D.-G.; Kang, H.-C.; Kim, J.; et al. Diterpenes from the roots of Oryza sativa L. and their inhibitory activity on NO production in LPS-stimulated RAW264.7 macrophages. Chem. Biodivers. 2015, 12, 1356–1364. [Google Scholar] [CrossRef]
- Kodama, O.; Li, W.X.; Tamogami, S.; Akatsuka, T. Oryzalexin S, a novel stemarane-type diterpene rice phytoalexin. Biosci. Biotechnol. Biochem. 1992, 56, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Tamogami, S.; Mitani, M.; Kodama, O.; Akatsuka, T. Oryzalexin S structure: A new stemarane-type rice plant phytoalexin and its biogenesis. Tetrahedron 1993, 49, 2025–2032. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Badger, T.M. Characterization of triterpene alcohol and sterol ferulates in rice bran using LC-MS/MS. J. Agric. Food Chem. 2003, 51, 3260–3267. [Google Scholar] [CrossRef]
- Luo, H.-F.; Li, Q.; Yu, S.; Badger, T.M.; Fang, N. Cytotoxic hydroxylated triterpene alcohol ferulates from rice bran. J. Nat. Prod. 2005, 68, 94–97. [Google Scholar] [CrossRef]
- Kuljanabhagavad, T.; Wink, M. Biological activities and chemistry of saponins from Chenopodium quinoa Willd. Phytochem. Rev. 2009, 8, 473–490. [Google Scholar] [CrossRef]
- Lerma-Garcia, M.J.; Herrero-Martinez, J.M.; Simo-Alfonso, E.F.; Mendoca, C.R.B.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Minatel, I.O.; Francisqueti, F.V.; Correa, C.R.; Lima, G.P.P. Antioxidant activity of γ-oryzanol: A complex network of interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [PubMed]
- Okahara, F.; Suzuki, J.; Hashizume, K.; Osaki, N.; Shimotoyodome, A. Triterpene alcohols and sterols from rice bran reduce postprandial hyperglycemia in rodents and humans. Mol. Nutr. Food Res. 2016, 60, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Yasukawa, K.; Yamaura, M.; Ukiya, M.; Kimura, Y.; Shimizu, N.; Arai, K. Triterpene alcohol and sterol ferulates from rice bran and their anti-inflammatory effects. J. Agric. Food Chem. 2000, 48, 2313–2319. [Google Scholar] [CrossRef] [PubMed]
- Verardo, V.; Gomez-Caravaca, A.M.; Marconi, E.; Segura-Carretero, A.; Garrido-Frenich, A.; Fernandez-Gutierrez, A. Determination of lipophilic and hydrophilic bioactive compounds in raw and parboiled rice bran. RSC Adv. 2016, 6, 50786. [Google Scholar] [CrossRef]
- Shu, X.-L.; Frank, T.; Shu, Q.-Y.; Engel, K.-H. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef] [PubMed]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamokkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Ahmad, A.; Lim, J.D.; Yu, C.Y.; Kim, J.S. Chemical constituents of rice (Oryza sativa) hulls and their herbicidal activity against duckweed (Lemna paucicostata Hegelm 381). Phytochem. Anal. 2006, 17, 36–45. [Google Scholar] [CrossRef]
- Kumar, M.S.S.; Ali, K.; Dahuja, A.; Tyagi, A. Role of phytosterols in drought stress tolerance in rice. Plant Physiol. Biochem. 2015, 96, 83–89. [Google Scholar] [CrossRef]
- Jung, Y.-J.; Park, J.-H.; Shrestha, S.; Song, M.-C.; Cho, S.; Lee, C.-H.; Han, D.; Baek, N.-I. Phytosterols from the rice (Oryza sativa) bran. J. Appl. Biol. Chem. 2014, 57, 175–178. [Google Scholar] [CrossRef]
- Ohnishi, M.; Fujino, Y. Novel glycolipids; cellobiosylsterol and cellotriosylsterol in rice bran. Agric. Biol. Chem. 1978, 42, 2423–2425. [Google Scholar]
- Ohnishi, M.; Fujino, Y. Structural study on new sterylglycosides in rice bran: Cellotetraosylsitosterol and cellopentaosylsitosterol. Agric. Biol. Chem. 1980, 44, 333–338. [Google Scholar]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O.; Turnbaugh, J.G. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Mahatheeranont, S.; Keawsa-ard, S.; Dumri, K. Quantification of the rice aroma compound, 2-acetyl-1-pyrroline, in uncooked Khao Dauk Mali 105 brown rice. J. Agric. Food Chem. 2001, 49, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Kaikavoosi, K.; Kad, T.D.; Zanan, R.L.; Nadaf, A.B. 2-Acetyl-1-pyrroline augmentation in scented indica rice (Oryza sativa L.) varieties through ∆1-pyrroline-5-carboxylate synthetase (P5CS) gene transformation. Appl. Biochem. Biotechnol. 2015, 177, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Ueno, K.; Teraishi, M.; Okumoto, Y.; Mori, N.; Ishihara, A. Induced phenylamide accumulation in response to pathogen infection and hormone treatment in rice (Oryza sativa). Biosci. Biotechnol. Biochem. 2018, 82, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Thi, H.L.; Lin, C.-H.; Smeda, R.J.; Leigh, N.D.; Wycoff, W.G.; Fritschi, F.B. Isolation and identification of an allelopathic phenylethylamine in rice. Phytochemistry 2014, 108, 109–121. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Miyagawa, H.; Wakasa, K. Induction of serotonin accumulation by feeding of rice striped stem borer in rice leaves. Plant Signal. Behav. 2008, 3, 714–716. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by YUCCA genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Tanaka, K.; Taniguchi, S.; Tamaoki, D.; Yoshitomi, K.; Akimitsu, K.; Gomi, K. Multiple roles of plant volatiles in jasmonate-induced defense response in rice. Plant Signal. Behav. 2014, 9, e29247. [Google Scholar] [CrossRef]
- Chung, I.-M.; Lim, Y.-H.; Ali, M.; Sultana, S.; Ahmad, A. Novel anthracene derivatives isolated from rice hulls of Oryza sativa and their growth inhibitory activity of radish seed. Bull. Korean Chem. Soc. 2006, 27, 995–1000. [Google Scholar] [CrossRef]
- Guo, H.-M.; Li, H.-C.; Zhou, S.-R.; Xue, H.-W.; Miao, X.-X. cis-12-Oxo-phytodiennoic acid stimulates rice defense response to a piercing-sucking insect. Mol. Plant 2014, 7, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Sookwong, P.; Murata, K.; Nakagawa, K.; Shibata, A.; Kimura, T.; Yamaguchi, M.; Kojima, Y.; Miyazawa, T. Cross-fertilization for enhancing tocotrienol biosynthesis in rice plants and QTL analysis of their F2 progenies. J. Agric. Food Chem. 2009, 57, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, M.J.; Lee, J.Y.; Kim, J.K.; Ha, S.-H.; Lim, S.-H. Molecular and biochemical analysis of two rice flavonoid 3′-hydroxylase to evaluate their roles in flavonoid biosynthesis in rice grain. Int. J. Mol. Sci. 2016, 17, 1549. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Okada, K.; Yamane, H.; Iwai, T.; Ohashi, Y. Analysis on blast fungus-responsive characters of a flavonoid phytoalexin sakuranetin; accumulation in infected rice leaves, antifungal activity and detoxification by fungus. Molecules 2014, 19, 11404–11418. [Google Scholar] [CrossRef] [PubMed]
- Rakwal, R.; Tamogami, S.; Kodama, O. Role of jasmonic acid as a signaling molecule in copper chloride-elicited rice phytoalexin production. Biosci. Biotechnol. Biochem. 1996, 60, 1046–1048. [Google Scholar] [CrossRef]
- Tamogami, S.; Rakwal, R.; Kodama, O. Phytoalexin production elicited by exogenously applied jasmonic acid in rice leaves (Oryza sativa L.) is under the control of cytokinins and ascorbic acid. FEBS Lett. 1997, 412, 61–64. [Google Scholar] [CrossRef]
- Nakazato, Y.; Tamogami, S.; Kawai, H.; Hasegawa, M.; Kodama, O. Methionine-induced phytoalexin production in rice leaves. Biosci. Biotechnol. Biochem. 2000, 64, 577–583. [Google Scholar] [CrossRef]
- Tamogami, S.; Shigeru, K.; Osamu, H.; Hirose, K.; Akatsuka, T. Pretilachlor [2-chloro-N-(2,6-diethylphenyl)-N-(2-propoxyethyl) acetamide]- and butachlor [N-(butoxymethl)-2-chloro-N-(2,6-diethylphenyl) acetamide]-induced accumulation of phytoalexin in rice (Oryza sativa) plants. J. Agric. Food Chem. 1995, 43, 1695–1697. [Google Scholar] [CrossRef]
- Tamogami, S.; Kodama, O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry 2000, 54, 689–694. [Google Scholar] [CrossRef]
- Plowright, R.A.; Grayer, R.J.; Gill, J.R.; Rahman, M.L.; Harborne, J.B. The induction of phenolic compounds in rice after infection by the stem nematode Ditylenchus angustus. Nematologica 1996, 42, 564–578. [Google Scholar]
- Kanno, H.; Hasegawa, M.; Kodama, O. Accumulation of salicylic acid, jasmonic acid and phytoalexins in rice, Oryza sativa, infested by the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Appl. Entomol. Zool. 2012, 47, 27–34. [Google Scholar] [CrossRef]
- Ogawa, S.; Miyamoto, K.; Nemoto, K.; Sawasaki, T.; Yamane, H.; Nojiri, H.; Okada, K. OsMYC2, an essential factor for JA-inductive sakuranetin production in rice, interacts with MYC2-like proteins that enhance its transactivation ability. Sci. Rep. 2017, 7, 40175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oritani, T.; Kiyota, H. Biosynthesis and metabolism of abscisic acid and related compounds. Nat. Prod. Rep. 2003, 20, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Heden, P.; Thomas, S.G. Gibberellin biosynthesis and its regulation. Biochem. J. 2012, 444, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, R.J. Unconvering the complex metabolic network underlying diterpenoid phytoalexin biosynthesis in rice and other cereal crop plants. Phytochemistry 2006, 67, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, T. Recent advances regarding diterpene cyclase genes in higher plants and fungi. Biosci. Biotechnol. Biochem. 2008, 72, 1168–1175. [Google Scholar] [CrossRef]
- Okada, K. The biosynthesis of isoprenoids and the mechanisms regulating it in plants. Biosci. Biotechnol. Biochem. 2011, 75, 1219–1225. [Google Scholar] [CrossRef]
- Okada, A.; Shimizu, T.; Okada, K.; Kuzuyama, T.; Koga, J.; Shibuya, N.; Nojiri, H.; Yamane, H. Elicitor induced activation of the methylerythritol phosphate pathway toward phytoalexins biosynthesis in rice. Plant Mol. Biol. 2007, 65, 177–187. [Google Scholar] [CrossRef]
- Miyamoto, K.; Fujita, M.; Shenton, M.F.; Shenton, M.R.; Akashi, S.; Sugawara, C.; Sakai, A.; Horie, K.; Hasegawa, M.; Kawide, H.; et al. Evolutionary trajectory of phytoalexin biosynthetic gene clusters in rice. Plant J. 2016, 87, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Toyomasu, T.; Goda, C.; Sakai, A.; Miyamoto, K.; Shenton, M.R.; Tomiyama, S.; Mitsuhashi, W.; Yamane, H.; Kurata, N.; Okada, K. Characterization of diterpene synthase genes in the wild rice species Oryza brachyatha provides evolutionary insight into rice phytoalexin biosynthesis. Biochem. Biophys. Res. Commun. 2018, 503, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Yamazaki, K.; Minoda, H.; Miyamoto, K.; Miyazaki, S.; Kawaide, H.; Yajima, A.; Nojiri, H.; Yamane, H.; Okada, K. In planta functions of cytochrome P450 monooxygenase genes in the phytocassane biosynthetic gene cluster on rice chromosome 2. Biosci. Biotechnol. Biochem. 2018, 82, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Cerezo, S.; Martinez-Montiel, N.; Garcia-Sanchez, J.; Perez-y-Terron, R.; Martinez-Contreras, R.D. Gibberellin biosynthesis and metabolism: A convergent route for plants fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Q.; Hillwig, M.L.; Peters, R.J. Picking sides: Distinct roles for CYP76M6 and CYP76M8 in rice oryzalexin biosynthesis. Biochem. J. 2013, 454, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, C.A.; Siebenmorgen, T.J. Nutraceutical concentrations within the bran of various rice kernel thickness fractions. Biosyst. Eng. 2004, 88, 453–460. [Google Scholar] [CrossRef]
- Matsuzuka, K.; Kimura, E.; Nakagawa, K.; Murata, K.; Kimura, T.; Miyazawa, T. Investigation of tocotrienol biosynthesis in rice (Oryza sativa L.). Food Chem. 2013, 140, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wakte, K.; Zanan, R.; Hinge, V.; Khandagale, K.; Nadaf, A.; Henry, R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (Oryza sativa L.): A status review. J. Sci. Food Agric. 2017, 97, 384–395. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the desense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef]
- Bradlbury, L.M.T.; Gillies, S.A.; Brushett, D.J.; Waters, D.L.E.; Henry, R.J. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 2008, 68, 439–449. [Google Scholar] [CrossRef] [Green Version]
- Dubouzet, J.G.; Matsuda, F.; Ishihara, A.; Miyagawa, H.; Wakasa, K. Production of indole alkaloids by metabolic engineering of the tryptophan pathway in rice. Plant Biotechnol. J. 2013, 11, 1103–1111. [Google Scholar] [CrossRef] [Green Version]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tiss. Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, I.; Carneiro, G.A.; Spadaro, D.; Garibaldi, A.; Gullino, M.L. Jamonic acid, abscisic acid, and salicylic acid are involved in the phytoalexin responses of rice to Fusarium fujikuroi, a high gibberellin producer pathogen. J. Agric. Food Chem. 2015, 63, 8134–8142. [Google Scholar] [CrossRef]
- Zhou, M.; Memelink, J. Jasmonate-responsive transcription factors regulating plant secondary metabolism. Biotechnol. Adv. 2016, 34, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Miyamoto, K.; Yamane, H.; Nishizawa, Y.; Minami, E.; Nojiri, H.; Okada, K. OsTGAP1 is responsible for JA-inducible diterpenoid phytoalexin biosynthesis in rice roots with biological impacts on allelopathic interaction. Physiol. Plantarum 2017, 161, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Tamogami, S.; Rakwal, R.; Kodama, O. Phytoalexin production by amino acid conjugates of jasmonic acid through induction of naringenin-7-O-methyltransferase, a key enzyme on phytoalexin biosynthesis in rice (Oryza sativa L.). FEBS Lett. 1997, 401, 239–242. [Google Scholar] [CrossRef]
- Miyamoto, K.; Enda, I.; Okada, T.; Sato, Y.; Watanabe, K.; Sakazawa, T.; Yumoto, E.; Shibata, K.; Asahina, M.; Lino, M.; et al. Jasmonoyl-L-isoleucine is required for the production of a flavonoid phytoalexin but not diterpenoid phytoalexins in ultraviolet-irradiated rice leaves. Biosci. Biotechnol. Biochem. 2016, 80, 1934–1938. [Google Scholar] [CrossRef]
- Ko, K.W.; Okada, K.; Koga, J.; Jikumaru, Y.; Nojiri, H.; Ymane, H. Effects of cytokinin on production of diterpenoid phytoalexins in rice. J. Pestic. Sci. 2010, 35, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Daw, B.D.; Zhang, L.H.; Wang, Z.Z. Salicylic acid enhances antifungal resistance to Magnaporthe grisea in rice plants. Aust. Plant Pathol. 2008, 37, 637–644. [Google Scholar] [CrossRef]
- Akagi, A.; Fukushima, S.; Okada, K.; Jiang, C.J.; Yoshida, R.; Nakayama, A.; Shimono, M.; Sugano, S.; Yamane, H.; Takatsuji, H. WRKY45-dependent priming of diterpeoid phytoalexin biosynthesis in rice and the role of cytokinin in triggering the reaction. Plant Mol. Biol. 2014, 86, 171–183. [Google Scholar] [CrossRef]
- Hamada, H.; Kurusu, T.; Nokajima, H.; Kiyoduka, M.; Yano, K.; Kuchitsu, K. Regulation of xylanase elicitor-induced expression of defense-related genes involved in phytoalexin biosynthesis by a cation channel OsTPC1 in suspension-cultured rice cells. Plant Biotechnol. 2014, 31, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Zheng, G.; Wang, S.; Gan, F. Effects of oligosaccharins on callus growth and saponin content of Panax notoginseng. Cell Res. 1992, 2, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Minami, E. Oligosaccharide signaling for defence responses in plant. Physiol. Mol. Plant Pathol. 2001, 59, 223–233. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, X.; Zhang, R.; Peng, Y.; Zhao, S.; Wu, J. Stimulation of saponin production in Panax ginseng hairy roots by two oligosaccharides from Paris polyphylla var. yunnanensis. Biotechnol. Lett. 2007, 29, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, P.; Xu, L.; Chen, Y.; Sui, P.; Zhou, L.; Li, J. Enhancement of diosgenin production in Dioscorea zingiberensis cell culture by oligosaccharide elicitor from its endophytic fungus Fusarium oxysporum Dzf17. Nat. Prod. Commun. 2009, 4, 1459–1462. [Google Scholar] [PubMed]
- Li, P.; Mao, Z.; Lou, J.; Li, Y.; Mou, Y.; Lu, S.; Peng, Y.; Zhou, L. Enhancement of diosgenin production in Dioscorea zingiberensis cell cultures by oligosaccharides from its endophytic fungus Fusarium oxysporum Dzf17. Molecules 2011, 16, 10631–10644. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shan, T.; Mou, Y.; Li, P.; Zhao, J.; Zhao, W.; Peng, Y.; Zhou, L.; Ding, C. Enhancement of palmarumycin C12 and C13 production in liquid culture of endophytic fungus Berkleasmium sp. Dzf12 by oligosaccharides from its host plant Dioscorea zingiberensis. Molecules 2012, 17, 3761–3773. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Luo, H.; Meng, J.; Sun, W.; Wang, X.; Lu, S.; Peng, Y.; Zhou, L. Effects of oligosaccharides from endophytic Fusarium oxysporum Dzf17 on activities of defense-related enzymes in Diosocrea zingiberensis suspension cell and seedling cultures. Electron. J. Biotechnol. 2014, 17, 156–161. [Google Scholar] [CrossRef]
- Ren, Y.-Y.; West, C.A. Elicitation of diterpene biosynthesis in rice (Oryza sativa L.) by chitin. Plant Physiol. 1992, 99, 1169–1178. [Google Scholar] [CrossRef]
- Yamada, A.; Shibuya, N.; Kodama, O.; Akatsuka, T. Induction of phytoalexins formation in suspension-cultured rice cells by N-acetylchitooligosaccharides. Biosci. Biotechnol. Biochem. 1993, 57, 405–409. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamada, A.; Hong, N.; Ogawa, T.; Ishii, T.; Shibuya, N. Differences in the recognition of glucan elicitor signals between rice and soybean: β-glucan fragments from the rice blast disease fungus Pyricularia oryzae that elicit phytoalexin biosynthesis in suspension-cultrued rice cells. Plant Cell 2000, 12, 817–826. [Google Scholar]
- Yamaguchi, T.; Maehara, Y.; Kodama, O.; Okada, M.; Matsumura, M.; Shibuya, N. Two purified oligosaccharide elicitors, N-acetylchitohepatose and tetraglucosyl glucitol, derived from Magnaporthe grisea cell walls, synergistically activate biosynthesis of phytoalexin in suspension-cultured rice cells. J. Plant Physiol. 2002, 159, 1147–1149. [Google Scholar] [CrossRef]
- Kaku, H.; Nishizawa, Y.; Ishii-Minami, N.; Akimoto-Tomiyama, C.; Dohmae, N.; Takio, K.; Minami, E.; Shibuya, N. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 11086–11091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouzai, Y.; Kaku, H.; Shibuya, N.; Minami, E.; Nishizawa, Y. Expression of the chimeric receptor between the chitin elicitor receptor CEBiP and the receptor-like protein kinase Pi-d2 leads to enhanced responses to the chitin elicitor and disease reistance against Magnaporthe oryzae in rice. Plant Mol. Biol. 2013, 81, 287–295. [Google Scholar] [CrossRef]
- Miyamoto, K.; Nishizawa, Y.; Minami, E.; Nojiri, H.; Yamane, H.; Okada, K. Overexpression of the bZIP transcription factor OsbZIP79 suppresses the production of diterpenoid phytoalexin in rice cells. J. Plant Physiol. 2015, 173, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.X.; Chen, J.H. The cerebrosides. Nat. Prod. Rep. 2003, 20, 509–534. [Google Scholar] [CrossRef]
- Umemura, K.; Ogawa, N.; Koga, J.; Iwata, M.; Usami, H. Elicitor activity of cerebroside, a sphingolipid elicitor, in cell suspension cultures of rice. Plant Cell Physiol. 2002, 43, 778–784. [Google Scholar] [CrossRef]
- Koga, J.; Yamauchi, T.; Shimura, M.; Ogawa, N.; Oshima, K.; Umemura, K.; Kikuchi, M.; Ogasawara, N. Cerebrosides A and C, sphingolipid elicitors of hypersensitive cell death and phytoalexin accumulation in rice plants. J. Biol. Chem. 1998, 273, 31985–31991. [Google Scholar] [CrossRef] [PubMed]
- Umemura, K.; Ogawa, N.; Yamauchi, T.; Iwata, M.; Shimura, M.; Koga, J. Cerebroside elicitors found in diverse phytopathogens activate defense responses in rice plants. Plant Cell Physiol. 2000, 41, 676–683. [Google Scholar] [CrossRef]
- Umemura, K.; Tanino, S.; Nagatsuka, T.; Koga, J.; Iwata, M.; Nagashima, K.; Amemiya, Y. Cerebroside elicitor confers resistance to Fusarium disease in various plant species. Phytopathology 2004, 94, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Koga, J.; Kubota, H.; Gomi, S.; Umemura, K.; Ohnishi, M.; Kono, T. Cholic acid, a bile acid elicitor of hypersensitive cell death, pathgenesis-related protein synthesis, and phytoalexin accumulation in rice. Plant Physiol. 2006, 140, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Jikumaru, Y.; Okada, A.; Okada, K.; Koga, J.; Umemura, K.; Minami, E.; Shibuya, N.; Hasegawa, M.; Kodama, O.; et al. Effects of a bile acid elicitor, cholic acid, on the biosynthesis of diterpenoid phytoalexins in suspension-cultured rice cells. Phytochemistry 2008, 69, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Sudo, E.; Itouga, M.; Yoshida-Hatanaka, K.; Ono, Y.; Sakakibara, H. Gene expression and sensitivity in response to copper stress in rice leaves. J. Exp. Bot. 2008, 59, 3465–3474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.G.; Wu, J.Y. Development and application of medicinal plant tissue cultures for production of drugs and herbal medicinals in China. Nat. Prod. Rep. 2006, 23, 789–810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-L.; Zhou, L.-G.; Wu, J.-Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Gandi, S.; Rao, K.; Chodisetti, B.; Giri, A. Elicitation of andrographolide in the suspension cultures of Andrographis paniculata. Appl. Biochem. Biotechnol. 2012, 168, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kastell, A.; Speiser, C.; Smetanska, I. Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Appl. Biochem. Biotechnol. 2013, 171, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Durango, D.; Quinones, W.; Torres, F.; Rosero, Y.; Gil, J.; Echeverri, F. Phytoalexin accumulation in Colombian bean varieties and aminosugars as elicitors. Molecules 2002, 7, 817–832. [Google Scholar] [CrossRef]
- Kodama, O.; Yamada, A.; Yamamoto, A.; Takemoto, T.; Akatsuka, T. Induction of phytoalexins with heavy metal ions in rice leaves. J. Pestc. Sci. 1988, 13, 615–617. [Google Scholar] [CrossRef]
- Kodama, O.; Suzuki, T.; Miyakawa, J.; Akatsuka, T. Ultraviolet-induced accumulation of phytoalexins in rice leaves. Agric. Biol. Chem. 1988, 52, 2469–2473. [Google Scholar]
- Kato-Noguchi, H.; Kujime, H.; Ino, T. UV-induced momilactone B accumulation in rice rhizosphere. J. Plant Physiol. 2007, 164, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, D.W.; Langcake, P.; Ride, J.P. Phytoalexin production in rice and its enhancement by a dichlorocyclopropane fungicide. Physiol. Plant Pathol. 1980, 17, 259–267. [Google Scholar] [CrossRef]
- Goufo, P.; Pereira, J.; Figureiredo, N.; Oliveira, M.B.P.P.; Carranca, C.; Rosa, E.A.S.; Trindade, H. Effect of elevated carbon dioxide (CO2) on phenolic acids, flavonoids, tocopherols, tocotrienols, γ-oryzanol and antioxidant capacities of rice (Oryza sativa L.). J. Cereal Sci. 2014, 59, 15–24. [Google Scholar] [CrossRef]
- Ejike, C.E.C.C.; Gong, M.; Udenigwe, C.C. Phytoalexins from the Poaceae: biosynthesis, function and prospects in food preservation. Food Res. Int. 2013, 52, 167–177. [Google Scholar] [CrossRef]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Imai, T.; Koga, J.; Okada, K.; Yamane, H.; Ohashi, Y. Phytoalexin accumulation in the interaction between rice and the blast fungus. Mol. Plant-Micro. Intrac. 2010, 23, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Fukami, A.; Matsuda, Y.; Nakajima, H.; Miyagawa, H. Accumulation of indole-3-acetic acid in rice sl mutant leaves infected with Bipolaris oryzae. J. Phytopathol. 2016, 164, 509–519. [Google Scholar] [CrossRef]
- Lu, H.; Luo, T.; Fu, H.; Wang, L.; Tan, Y.; Huang, J.; Wang, Q.; Ye, G.; Gatehouse, A.M.R.; Lou, Y.; et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef]
- Kyndt, T.; Denil, S.; Bauters, L.; Van Criekinge, W.; De Meyer, T. Systemic suppression of the shoot metabolism upon rice root nematode infection. PLoS ONE 2014, 9, e106858. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, F.; Cao, X.; Chen, M.; Ye, G.; Wei, C.; Li, Y. The rice dwarf virus P2 protein interacts with ent-kaurene oxidases in vivo, leading to reduced biosynthesis of gibberellins and rice dwarf symptoms. Plant Physiol. 2005, 139, 1935–1945. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Bamyard grass-induced rice allelopathy and momilactone B. J. Plant Physiol. 2011, 168, 1016–1020. [Google Scholar] [CrossRef]
- Dayan, F.E. Factors modulating the levels of the allelochemical sorgolene in Sorghum bicolor. Planta 2006, 224, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Boiteau, R.M.; Hoyt, D.W.; Nicora, C.D.; Kinmonth-Schultz, H.A.; Ward, J.K.; Bingol, K. Structure elucidation of unknown metabolites in metabolomics by combined NMR and MS/MS prediction. Metabolites 2018, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clement, C.; Jeandet, P. Molecular engineering of resveratrol in plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Jeandet, P.; Courot, E.; Clement, C.; Ricord, S.; Crouzet, J.; Aziz, A.; Cordelier, S. Molecular engineering of phytoalexins in plants: Benefits and limitations for food and agriculture. J. Agric. Food Chem. 2017, 65, 2643–2644. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, S.-W.; Jung, K.-H.; Hahn, T.-R.; Cho, M.-H. Transcriptomic analysis of UV-treated rice leaves revrals UV-induced phytoalexin biosynthesis pathways and their regulatory networks in rice. Phytochemistry 2013, 96, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Stark-Lorenzen, P.; Nelke, B.; Hanbler, G.; Muhlbach, H.P.; Thomzik, J.E. Transfer of a grapevine stilbene synthase gene to rice (Oryza sativa L.). Plant Cell Rep. 1997, 16, 668–673. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, J.; Guo, B.; Duan, J.; Che, C.-T. Momilactone and related diterpenoids as potential agricultural chemicals. J. Agric. Food Chem. 2018, 66, 7859–7872. [Google Scholar] [CrossRef]

























| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| p-Hydroxybenzaldehyde (1) | Husk and bran | - | [24] |
| Bran | Antioxidant activity | [31] | |
| p-Hydroxybenzoic acid (2) | Root exudate | Allelopathic effect | [30] |
| Husk and bran | - | [24] | |
| p-Hydroxy methyl benzoate glucoside (3) | Bran | Antioxidant activity | [31] |
| p-Hydroxy phenyl acetaldehyde (4) | Husk and bran | - | [24] |
| p-Hydroxy phenyl acetic acid (5) | Husk and bran | - | [24] |
| 2-Hydroxy 5-[(3S)-3-hydroxybutyl] phenyl β-d-glucoside (HHPG) (6) | Brans of purple rice | Inhibitory activity on tunicamycin-induced retinal damage | [32] |
| Caffeic acid (7) | Endosperm and bran/embryo of indica variety | Antioxidant activity | [20] |
| Root exudate | Allelopathic effect | [30] | |
| Husk and bran | - | [24] | |
| Methyl caffeate (8) | Bran | Antioxidant activity | [31] |
| Caffeoyl quinic acid methyl ester (9) | Grains of brown rice | - | [33] |
| Protocatechuic acid (10) | Endosperm and bran/embryo of indica variety | Antioxidant activity | [20] |
| Chlorogenic acid (11) | Endosperm and bran/embryo of indica variety | Antioxidant activity | [20] |
| Cinnamic acid (12) | Husk and bran | - | [24] |
| o-Coumaric acid (13) | Endosperm and bran/embryo of indica variety | Antioxidant activity | [20] |
| Root exudate | Allelopathic effect | [30] | |
| m-Coumaric acid (14) | Grains | - | [23] |
| p-Coumaric acid (15) | Grains | - | [23] |
| Grains of brown rice | - | [33] | |
| 3-O-p-Coumaroyl quinic acid (16) | Grains of brown rice | - | [33] |
| Leaves | - | [34] | |
| trans-Ferulic acid (17) | Endosperm and bran/embryo of indica variety | Antioxidant activity | [20] |
| Grains | - | [23] | |
| Black rice bran | Antioxidant activity | [25] | |
| Husk and bran | - | [24] | |
| Bran | Antioxidant activity | [31] | |
| trans-Ferulic acid methyl ester (18) | Bran | Antioxidant activity | [31] |
| cis-Ferulic acid (19) | Bran | Antioxidant activity | [31] |
| cis-Ferulic acid methyl ester (20) | Bran | Antioxidant activity | [31] |
| 1,3-O-Diferuloylglycerol (21) | Leaves | - | [34] |
| 1-O-Feruloyl-β-d-glucose (22) | Leaves | - | [34] |
| 3-O-Feruloylquinic acid (23) | Leaves | - | [34] |
| Gallic acid (24) | Endosperm and bran/embryo of indica variety | Antioxidant activity | [20] |
| Husk and bran | - | [24] | |
| m-Salicylic acid (25) | Grains of brown rice | - | [33] |
| Salicylic acid 2-O-β-d-glucopyranoside (26) | Leaves | - | [34] |
| Sinapic acid (27) | Grains | - | [23] |
| 1-O-Sinapoyl-β-d-glucose (28) | Leaves | - | [34] |
| Syringaldehyde (29) | Grains of brown rice | - | [33] |
| Syringic acid (30) | Endosperm and bran/embryo of indica variety | Antioxidant activity | [20] |
| Root exudate | Allelopathic effect | [30] | |
| Husk and bran | - | [24] | |
| Grains of brown rice | - | [33] | |
| Vanillic aldehyde (31) | Bran | Antioxidant activity | [31] |
| Vanillic acid (32) | Root exudate | Allelopathic effect | [30] |
| Husk and bran | - | [24] | |
| Grains of brown rice | - | [33] |
| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| Flavones | |||
| Apigenin 6-C-α-l-arabinosyl-8-C-β-l-arabinoside (33) | Leaves | - | [53] |
| Chrysoeriol 7-O-rutinoside (34) | Grains of brown rice | - | [33] |
| Chrysoeriol 6-C-α-l-arabinosyl-8-C-β-l-arabinoside (35) | Leaves | - | [53] |
| 5,7,4′-Trihydroxy-3′,5′-dimethoxyflavone (36) | Leaves | Allelopathic activity; antifungal activity | [54,55] |
| Seedlings | Allelopathic activity | [41] | |
| 5,4′-Dihydroxy-3′,5′-dimethoxy-7-O-β-glucopyranosylflavone (37) | Seedlings | - | [41] |
| 7,4′-Dihydroxy-3′,5′-dimethoxy-5-O-β-glucopyranosylflavone (38) | Seedlings | - | [41] |
| Isoscoparin 2-O-(6-(E)-feruloyl)-glucopyranoside (39) | Leaves | - | [53] |
| Isoscoparin 2″-O-(6‴-(E)-p-coumaroyl)-glucopyranoside (40) | Leaves | - | [53] |
| Isovitexin 2″-O-(6‴-(E)-feruloyl)-glucopyranoside (41) | Leaves | - | [53] |
| Isovitexin 2″-O-(6‴-(E)-p-coumaroyl)-glucopyranoside (42) | Leaves | - | [53] |
| Isoorientin 7,3′-dimethyl ether (43) | Leaves | - | [53] |
| luteolin 6-C-(2″-O-β-d-glucopyranosyl)-α-l-arabinoside (44) | Leaves | - | [53] |
| Schaftoside (45) | Leaves | Antifeedant activity | [35] |
| Isoschaftoside (46) | Leaves | Antifeedant activity | [35] |
| Swertisin (47) | Leaves | - | [53] |
| Tricin (48) | Bran | DPPH radical scavenging activity | [56] |
| Tricin 7-O-β-d-glucopyranoside (49) | Leaves | - | [53] |
| Tricin 5-O-β-d-glucopyranoside (50) | Leaves | - | [53] |
| Tricin 7-O-rutinoside (51) | Leaves | - | [53] |
| Tricin 7-O-neohesperidoside (52) | Leaves | - | [53] |
| Tricin 7-O-(2″-O-β-d-glucopyranosyl)-β-d-glucuronopyranoside (53) | Leaves | - | [53] |
| Tricin 7-O-(6″-O-malonyl)-β-d-glucopyranoside (54) | Leaves | - | [53] |
| Tricin 7-O-(6″-(E)-sinapoyl)-β-d-glucopyranoside (55) | Leaves | - | [53] |
| Tricin 4′-O-(threo-β-syringylglyceryl) ether 7″-O-β-d-glucopyranoside (56) | Leaves | - | [53] |
| Tricin 4′-O-(erythro-β-guaiacylglyceryl) ether (57) | Bran | DPPH radical scavenging activity | [56] |
| Bran | Cytotoxicity and apoptosis induction in multiple tumor cells | [38] | |
| Tricin 4′-O-(threo-β-guaiacylglyceryl) ether (58) | Bran | DPPH radical scavenging activity | [56] |
| Bran | Cytotoxicity and apoptosis induction in multiple tumor cells | [38] | |
| Tricin 4′-O-(erythro-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranoside (59) | Leaves | - | [53] |
| Tricin 4′-O-(threo-β-guaiacylglyceryl) ether 7-O-β-d-glucopyranoside (60) | Leaves | - | [53] |
| Tricin 4′-O-(erythro-β-guaiacylglyceryl) ether 7″-O-β-d-glucopyranoside (61) | Leaves | - | [53] |
| Tricin 4′-O-(threo-β-guaiacylglyceryl) ether 7″-O-β-d-glucopyranoside (62) | Leaves | - | [53] |
| Tricin 4′-O-(erythro-β-guaiacylglyceryl) ether 9″-O-β-d-glucopyranoside (63) | Leaves | - | [53] |
| Tricin 4′-O-(threo-β-4-hydroxyphenylglyceryl) ether (64) | Leaves | - | [53] |
| Tricin 7-O-rutinoside (65) | Grains of brown rice | - | [33] |
| Flavonols | |||
| Brassicin (66) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| Brassicin-4′-O-β-d-glucopyranoside (67) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| Isorhamnetin-4′-O-β-d-glucopyranoside (68) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| Isorhamnetin-7-O-β-d-cellobioside (69) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| Kaempferol (70) | Husk and bran | - | [24] |
| Myricetin (71) | Rice flour | - | [57] |
| Quercetin (72) | Rice flour | - | [57] |
| Quercetin 3-O-glucoside (73) | Rice flour | - | [57] |
| Quercetin 3-O-galactoside = Hyperoside (74) | Rice flour | - | [57] |
| Qucertin 3-O-rutinoside = Rutin (75) | Rice flour | - | [57] |
| Syringetin 3-O-β-d-glucopyranoside (76) | Leaves | - | [53] |
| Syringetin 3-O-rutinoside (77) | Leaves | - | [53] |
| Flavanones | |||
| Hesperidin (78) | Rice flour | - | [57] |
| Naringenin (79) | Leaves | - | [42] |
| - | Antifungal activity | [43,44] | |
| Rice flour | - | [57] | |
| Rice fungal pathogen | - | [44,45] | |
| Naringenin 7-O-β-d-xylopyranoside (80) | Rice fungal pathogen | - | [45] |
| Sakuranetin (81) | Leaves | Antifungal activity | [43,44] |
| Leaves | Antibacterial and antifungal activities | [58] | |
| Leaves | Anti-Helicobacter pylori activity | [49] | |
| - | Antileishmanial and antitrypanosomal activities | [50] | |
| - | Antioxidant activity | [42] | |
| - | Anti-inflammatory activity | [47] | |
| - | Anti-mutagenic activity | [48] | |
| - | Induction of adipogenesis in 3T3-L1 cells | [46] | |
| - | Induction of melanogenesis in B16BL6 melanoma cells | [51] | |
| Sakuranetin 4′-O-β-d-xylopyranoside (82) | Rice fungal pathogen | - | [45] |
| Sternbin (83) | Rice fungal pathogen | - | [44] |
| Flavanonols | |||
| 3′-O-Methyltaxifolin (84) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| 3′-O-Methyltaxifolin-7-O-β-d-glucopyranoside (85) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| 3′-O-Methyltaxifolin-4′-O-β-d-glucopyranoside (86) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| 3′-O-Methyltaxifolin-5-O-β-d-glucopyranoside (87) | Grains of transgenic japonica | Radical scavenging activity | [39] |
| Flavanols | |||
| Catechin (88) | Rice flour | - | [57] |
| Epicatechin (89) | Rice flour | - | [57] |
| Anthocyanins | |||
| Cyanidin (90) | Bran | - | [59] |
| Black rice kernels | Antioxidant activity | [52] | |
| Cyanidin 3-O-gentiobioside (91) | Bran | - | [60] |
| - | Inhibitory activity on tunicamycin-induced retinal damage | [32] | |
| Cyanidin 3-O-glucoside (92) | Bran | - | [60] |
| Inhibitory activity on tunicamycin-induced retinal damage | [32] | ||
| Cyanidin 3-O-rutinoside (93) | Kernels | - | [61] |
| Cyanidin 3-O-sambubioside (94) | Black rice kernels | Antioxidant activity | [52] |
| Cyanidin 3,5-O-diglucoside (95) | Kernels | - | [61] |
| Delphinidin (96) | Bran | - | [59] |
| Malvidin (97) | Bran | - | [59] |
| Pelargonidin (98) | Bran | - | [59] |
| Pelargonidin 3,5-O-diglucoside (99) | Pigmented rice | Antioxidant activity | [2] |
| Peonidin (100) | Black rice kernels | Antioxidant activity | [52] |
| Peonidin 3-O-glucoside (101) | Bran | - | [60] |
| - | Inhibitory activity on tunicamycin-induced retinal damage | [32] | |
| Black rice kernels | Antioxidant activity | [52] |
| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| Camphene (102) | Bran | - | [65] |
| Camphor (103) | Bran | - | [65] |
| Carveol (104) | Bran | - | [65] |
| 1,4-Cineol (105) | Bran | - | [65] |
| Fenchyl acetate (106) | Bran | - | [65] |
| (S)-Limonene (107) | Leaves | - | [66] |
| Bran | - | [65] | |
| Seedlings | Antibacterial activity on Xoo | [63] | |
| Linalool (108) | Leaves | - | [66] |
| Leaves | Resistance induction to Xoo | [64] | |
| cis-Linalool oxide (109) | Bran | - | [65] |
| trans-Linalool oxide (110) | Bran | - | [65] |
| Myrcene (111) | Seedlings | - | [63] |
| Bran | - | [65] | |
| trans-β-Ocimene (112) | Bran | - | [65] |
| α-Pinene (113) | Seedlings | - | [63] |
| β-Pinene (114) | Bran | - | [65] |
| Sabinene (115) | Seedlings | - | [63] |
| Bran | - | [65] | |
| α-Terpinene (116) | Seedlings | - | [63] |
| γ-Terpinene (117) | Leaves | Antibacterial activity on Xoo | [62] |
| Terpinen-4-ol (118) | Bran | - | [65] |
| α-Thujene (119) | Seedlings | - | [63] |
| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| Abscisic acid (120) | Whole rice plant | Regulation of growth and development | [69] |
| (Z)-α-Bergamotene (121) | Leaves | - | [66] |
| β-Bisabolene (122) | Bran | - | [65] |
| (E)-γ-Bisabolene (123) | Leaves | - | [66] |
| α-Cadinene (124) | Leaves | - | [66] |
| β-Caryophyllene (125) | Leaves | - | [66] |
| Bran | - | [65] | |
| α-Copaene (126) | Leaves | - | [66] |
| Bran | - | [65] | |
| Seedlings | - | [63] | |
| α-Curcumene (127) | Leaves | [66] | |
| γ-Curcumene (128) | Leaves | [66] | |
| Cyclosativene (129) | Seedlings | - | [63] |
| α-Elemene (130) | Bran | - | [65] |
| β-Elemene (131) | Seedlings | - | [63] |
| (E)-β-Farnesene (132) | Leaves | - | [68] |
| Germacrene D (133) | Leaves | [66] | |
| α-Gurjunene (134) | Bran | - | [65] |
| β-Gurjunene (135) | Leaves | [66] | |
| α-Humulene (136) | Leaves | [66] | |
| Italicene (137) | Leaves | [66] | |
| γ-Muurolene (138) | Leaves | [66] | |
| (E)-Nerolidol (139) | Leaves | Antibacterial activity against Xoo | [68] |
| 7-epi-α-Selinene (140) | Bran | - | [65] |
| Valencene (141) | Leaves | - | [66] |
| Viridiflorene (142) | Leaves | - | [66] |
| α-Ylangene (143) | Bran | - | [65] |
| α-Zingiberene (144) | Leaves | - | [66] |
| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| Phytohormone gibberellins | |||
| Gibberellin A1 (145) | Whole plant | Growth-promoting activity | [70] |
| Gibberellin A4 (146) | Whole plant | Growth-promoting activity | [70] |
| Gibberellin A19 (147) | Whole plant | Growth-promoting activity | [70] |
| Pimaradiene-type diterpenoids | |||
| Momilactone A (148) | Coleoptiles | Antifungal activity | [71] |
| Bran | Growth inhibitory activity on rice roots | [90] | |
| Bran | Inhibitory activities on seed germination and growth of barnyard grass | [93] | |
| Root exudates | Allelopathy effect | [91] | |
| Momilactone B (149) | Coleoptiles | Antifungal activity | [71] |
| Seedlings | Growth inhibitory activity on rice roots | [90,92] | |
| Seedlings | Allelopathic effects | [40] | |
| Bran | Inhibitory activities on seed germination and growth of barnyard grass | [93] | |
| Root exudates | Allelopathy effect | [91] | |
| Momilactone C (150) | Bran | Weak growth inhibitory activity | [94] |
| Momilactone D (151) | Roots | - | [95] |
| Momilactone E (152) | Roots | - | [95] |
| 9β-Pimara-7,15-diene-3β,6β,19-triol (153) | Leaves | Weak antifungal activity | [72] |
| ent-Sandaracopimaradiene-type diterpenoids | |||
| ent-15,16-Epoxy-2,3-dihydroxy- kaurane (154) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [85] |
| ent-2,3,15-Trihydroxy- kaurane (155) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [85] |
| ent-15,16-Epoxy-kauran-3-one (156) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [85] |
| ent-15,16-Epoxy-kauran-2,3-dione = Oryzadione (157) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [86] |
| ent-15,16-Epoxy-3β-hydroxy-kauran-2-one (158) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [86] |
| ent-15,16-Epoxy-3-oxa-kauran-2-one (159) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [86] |
| ent-15,16-Epoxy-3β-myristoyloxy-kauran-2-one (160) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [86] |
| ent-15,16-Epoxy-3α-palmitoyloxy-kauran-2-one (161) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [86] |
| ent-15,16-Epoxy-3β-palmitoyloxy-kauran-2-one (162) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [86] |
| Oryzalexin A (163) | Leaves | Inhibitory activity on spore germination and germ tube growth of Ochrobactrum oryzae | [73,76] |
| Roots | - | [95] | |
| Oryzalexin B (164) | Leaves | Inhibitory activity on spore germination and germ tube growth of O. oryzae | [75,76] |
| Oryzalexin C (165) | Leaves | Inhibitory activity on spore germination and germ tube growth of O. oryzae | [75,76] |
| Oryzalexin D(166) | Leaves | Inhibitory activity on spore germination of Magnaporthe Oryzae | [77] |
| Oryzalexin E (167) | Leaves | Inhibitory activity on spore germination of M. Oryzae | [78] |
| Oyzalexin F (168) | Leaves | Antimicrobial activity | [79] |
| Oryzalic acid A (169) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [88] |
| Oryzalic acid B = ent-15-Hydroxy-2,3-secokauren- 2,3-dioic acid (170) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [85] |
| Oryzalide A = ent-15,16-Epoxy-1α-hydroxy-2-oxa-kauran-3-one (171) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [87,88] |
| Oryzalide B (172) | Leaves of a bacterial leaf blight-resistant cultivar | Antibacterial activity | [88] |
| Sandaracopimaradien-3-one (173) | Roots | - | [95] |
| Stemarene-type diterpenoids | |||
| Oryzalexin S (174) | Leaves | Antifungal activity | [96,97] |
| Stemar-13-en-2α-ol (175) | Leaves | Weak antifungal activity | [72] |
| ent-Cassadiene-type diterpenoids | |||
| Phytocassane A (176) | Leaves infected with M. oryzae; stems infected with Rhizoctonia Solani | Antifungal activity | [80] |
| Phytocassane B (177) | Leaves infected with M. oryzae; stems infected with R. Solani | Antifungal activity | [80] |
| Phytocassane C (178) | Leaves infected with M. oryzae; stems infected with R. Solani | Antifungal activity | [80] |
| Phytocassane D (179) | Leaves infected with M. oryzae; stems infected with R. Solani | Antifungal activity | [80] |
| Phytocassane E (180) | Cultured rice cells | Inhibition activity on spore germination and germ tube growth of M. oryzae | [81] |
| Phytocassane F (181) | Leaves | Antifungal activity | [72] |
| Casbene-type diterpenoids | |||
| 5-Deoxo-ent-10-oxodepressin (182) | Leaves | Antifungal activity | [83] |
| 5-Dihydro-ent-10-oxodepressin (183) | Leaves | Antifungal activity | [83] |
| ent-10-Oxodepressin (184) | Leaves | Antifungal activity | [84] |
| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| Citrostadienol (185) | Bran | - | [105] |
| Bran | Anti-inflammatory activity | [104] | |
| Citrostadienol cis-ferulate (186) | Bran | Anti-inflammatory activity | [104] |
| Citrostadienol trans-ferulate (187) | Bran | Anti-inflammatory activity | [104] |
| (24S)-Cycloart-25-ene-3β,24-diol-3β-trans-ferulate (188) | Bran | Moderate cytotoxic activity | [99] |
| (24R)-Cycloart-25-ene-3β,24-diol-3β-trans-ferulate (189) | Bran | Moderate cytotoxic activity | [99] |
| Cycloart-23Z-ene-3β,25-diol-3β-trans-ferulate (190) | Bran | Moderate cytotoxic activity | [99] |
| Cycloartenol (191) | Bran | - | [105] |
| Bran | Lowering postpradial hyperglyceimia | [103] | |
| Cycloartenol trans-caffeate (192) | Seeds | - | [106] |
| Cycloartenol cis-ferulate (193) | Bran | - | [98] |
| Cycloartanol trans-ferulate (194) | Bran | - | [98] |
| Bran | Moderate cytotoxic activity | [99] | |
| 24-Methylene cycloartanol (195) | Bran of black non-glutinous rice | Anti-cancer activity | [107] |
| Bran | Lowering postpradial hyperglyceimia | [103] | |
| 24-Methylene cycloartanol cis-ferulate (196) | Bran | - | [98] |
| Bran | Anti-inflammatory activity | [104] | |
| 24-Methylene cycloartanol trans-ferulate (197) | Bran | - | [98] |
| Bran | Moderate cytotoxic activity | [99] | |
| Cycloeucalenol (198) | Bran of black non-glutinous rice | Anti-cancer activity | [107] |
| Cycloeucalenol cis-ferulate (199) | Bran | Antioxidant activity | [31] |
| Cycloeucalenol trans-ferulate (200) | Bran | - | [98] |
| Bran | Anti-inflammatory activity | [104] | |
| Bran | Antioxidant activity | [31] | |
| Gramisterol (201) | Bran of black non-glutinous rice | Anti-cancer activity | [107] |
| Gramisterol cis-ferulate (202) | Bran | Anti-inflammatory activity | [104] |
| Gramisterol trans-ferulate (203) | Bran | Anti-inflammatory activity | [104] |
| Lanast-7,9(11)-dien-3α,15α-diol-3α-d-glucofuranoside (204) | Hulls | Herbicidal activity | [108] |
| Lupeol (205) | Bran of black non-glutinous rice | Anti-cancer activity | [107] |
| Lupenone (206) | Bran of black non-glutinous rice | Anti-cancer activity | [107] |
| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| ∆5-Avenasterol (207) | Germinating seeds | - | [106] |
| ∆7-Avenasterol (208) | Germinating seeds | - | [106] |
| Campestanol (209) | Germinating seeds | - | [106] |
| Campestanol trans-ferulate (210) | Bran | - | [98] |
| ∆7-Campestenol (211) | Germinating seeds | - | [106] |
| Campesterol (212) | Bran | - | [107] |
| Seedlings | Drought stress tolerance | [109] | |
| Campesterol trans-caffeate (213) | Bran | - | [98] |
| ∆7-Campesterol trans-ferulate (214) | Bran | - | [99] |
| Campesterol trans-ferulate (215) | Bran | - | [99] |
| Cholesterol (216) | Germinating seeds | - | [106] |
| 24-Methyl cholesterol cis-ferulate (217) | Bran | Anti-inflammatory activity | [105] |
| 24-Methylene cholesterol cis-ferulate (218) | Bran | Anti-inflammatory activity | [104] |
| 24-Methylene cholesterol trans-ferulate (219) | Bran | - | [98] |
| Bran | Anti-inflammatory activity | [104] | |
| 24-Methylene ergosta-5-en-3β-ol (220) | Bran | - | [107] |
| 24-Methylene ergosta-7-en-3β-ol (221) | Bran | - | [107] |
| Fucosterol (222) | Bran | - | [107] |
| Schleicheol 2 (223) | Bran | - | [110] |
| Sitostanol (224) | Germinating seeds | - | [106] |
| Sitosterol = β-Sitosterol (225) | Bran | - | [105,107] |
| Seedlings | Drought stress tolerance | [109] | |
| 7α-Hydroxy sitosterol (226) | Bran | - | [110] |
| 7β-Hydroxy sitosterol (227) | Bran | - | [110] |
| Sitosterol cis-ferulate (228) | Bran | - | [98] |
| Bran | Anti-inflammatory activity | [104] | |
| Sitosterol trans-ferulate (229) | Bran | - | [98] |
| ∆7-Sitosterol trans-ferulate (230) | Bran | - | [98] |
| d-Glucopyranosyl-(β1→4)-d-glucopyranosyl-(β1→3′)-β-sitosterol (231) | Bran (Hulls) | - | [111] |
| d-Glucopyranosyl-(β1→3)-d-glucopyranosyl-(β1→3′)-β-sitosterol (232) | Bran (Hulls) | - | [111] |
| d-Glucopyranosyl-(β1→4)-d-glucopyranosyl-(β1→4)-d-glucopyranosyl-(β1→3′)-β-sitosterol (233) | Bran (Hulls) | - | [111] |
| Cellotetraosylsitosterol (234) | Bran | - | [112] |
| Cellopentaosylsitosterol (235) | Bran | - | [112] |
| Stigmastanol cis-ferulate (236) | Bran | Anti-inflammatory activity | [104] |
| Stigmastanol trans-ferulate (237) | Bran | [98] | |
| Bran | Anti-inflammatory activity | [104] | |
| Stigmastanol-3β-p-butanoxy dihydrocoumaroate (238) | Hulls | Weak herbicidal activity | [108] |
| Stigmastanol-3β-p-glyceroxy dihydrocoumaroate (239) | Hulls | - | [108] |
| ∆7-Stigmastenol (240) | Germinating seeds | - | [106] |
| Stigmasterol (241) | Bran | - | [105,107] |
| Seedlings | Drought stress tolerance | [109] | |
| Stigmasterol cis-ferulate (242) | Bran | Anti-inflammatory activity | [104] |
| Stigmasterol trans-ferulate (243) | Bran | - | [98] |
| Bran | Anti-inflammatory activity | [104] |
| Name | Rice Part Used for Isolation | Biological Activity and function | Ref. |
|---|---|---|---|
| N-Feruloylagmatine (244) | Leaves | Antimicrobial activity | [116] |
| N-Feruloylputrescine (245) | Leaves | Antimicrobial activity | [116] |
| Kynurenic acid (246) | Leaves | - | [34] |
| Lycoperodine-1 (247) | Leaves | - | [34] |
| 2-Acetyl-1-pyrroline (248) | Grains | - | [113] |
| N-Benzoylserotonin (249) | Leaves | Antimicrobial activity | [116] |
| N-Benzoyltryptamine (250) | Leaves | Antimicrobial activity | [116] |
| Leaves | Antibacterial activity | [58] | |
| N-Benzoyltyramine (251) | Leaves | Antimicrobial activity | [116] |
| N-trans-Cinnamoylserotonin (252) | Leaves | Antimicrobial activity | [116] |
| N-trans-Cinnamoyltryptamine (253) | Leaves | Antimicrobial activity | [116] |
| Leaves | Antibacterial activity | [58] | |
| N-trans-Cinnamoyltyramine (254) | Whole rice plant | Allelopathic activity; antifungal activity | [117] |
| Leaves | Antibacterial activity | [58] | |
| N-p-Coumaroylserotonin (255) | Leaves | Antimicrobial activity | [116] |
| Leaves | Antibacterial activity | [58] | |
| N-Feruloylserotonin (256) | Leaves | Antimicrobial activity | [116] |
| N-Feruloyltryptamine (257) | Leaves | - | [118] |
| Indole 3-acetic acid (258) | Whole rice plant | Regulation on growth and development | [119] |
| Serotonin = 5-Hydroxytryptamine (259) | Leaves | - | [118] |
| Tryptamine (260) | Leaves | - | [118] |
| Name | Rice Part Used for Isolation | Biological Activity and Function | Ref. |
|---|---|---|---|
| (E,E)-2,4-Heptadienal (261) | Whole phants | Antibacterial and antifungal activities, toxic to rice plants | [120] |
| (Z)-3-Hexen-1-ol (262) | Leaves | - | [66] |
| Orizaanthracenol = 1-Methoxyanthracen-2-ol (263) | Hulls | Strong inhibitory activity in seed germination of radish | [121] |
| 1-Hydroxy-7-((2S,3R,4R,5S)-2″,3″,4″-trihydroxy-5″-(hydroxymethyl)tetrahydro-2H-pyran-1-yloxy)anthracen-2-yl 3′,7′-dimethyloctanoate (264) | Hulls | Weak inhibitory activity in seed germination of radish | [121] |
| 1-Hydroxy-7-((2S,3R,4R,5S)-2″,3″,4″-trihydroxy-5″-(hydroxymethyl)tetrahydro-2H-pyran-1-yloxy)anthracen-2-yl 3′,7′,11′,15′,19′-pentamethyltricosanoate (265) | Hulls | Weak inhibitory activity in seed germination of radish | [121] |
| (5S)-5-(Acetyloxy)-3-(1-methylenthyl)-2-cyclohexen-1-one = 3-Isopropyl-5-acetoxycyclohexene-2-one-1 (266) | Leaves | Allelopathic activity | [55] |
| Seedlings | Allelopathic effects | [40] | |
| cis-12-oxo-Phytodienoic acid (267) | Whole plants | Inducible anti-insect activity | [122] |
| 1-Phenyl-2-hydroxy-3,7-dimethyl-11-aldehydic-tetradecane-2β-d-glucopyranoside (268) | Hulls | Herbicidal activity | [108] |
| α-Tocopherol (269) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
| β-Tocopherol (270) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
| γ-Tocopherol (271) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
| δ-Tocopherol (272) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
| α-Tocotrienol (273) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
| β-Tocotrienol (274) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
| γ-Tocotrienol (275) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
| δ-Tocotrienol (276) | Bran | Antioxidative, antihypercholesterolemic, anticancer, neuroprotective activities | [123] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, Y.; Dang, P.; Zhao, S.; Lai, D.; Zhou, L. Rice Secondary Metabolites: Structures, Roles, Biosynthesis, and Metabolic Regulation. Molecules 2018, 23, 3098. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123098
Wang W, Li Y, Dang P, Zhao S, Lai D, Zhou L. Rice Secondary Metabolites: Structures, Roles, Biosynthesis, and Metabolic Regulation. Molecules. 2018; 23(12):3098. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123098
Chicago/Turabian StyleWang, Weixuan, Yuying Li, Pengqin Dang, Siji Zhao, Daowan Lai, and Ligang Zhou. 2018. "Rice Secondary Metabolites: Structures, Roles, Biosynthesis, and Metabolic Regulation" Molecules 23, no. 12: 3098. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules23123098