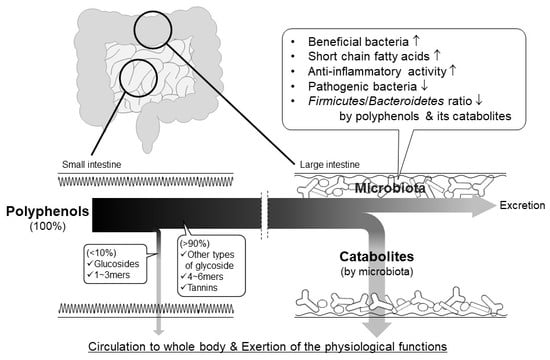
Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols
Abstract
:1. Introduction
1.1. Classification
1.2. Background of Polyphenol Research
1.3. Interest in the Actions of Intestinal Microbiota
2. Hydrolysis and Absorption of Polyphenols in the Stomach and Small Intestine
2.1. Stability of Polyphenols in the Stomach
2.2. Intestinal Absorption of Sugar-Bound Polyphenols
2.3. Intestinal Absorption of Monomeric Epicatechin and Its Related Monomeric Flavan-3-ols
2.4. Intestinal Absorption of Oligomeric Procyanidins
2.5. Hydrolysis of Tannins in the Digestive Tract
3. Decomposition and Metabolism by Intestinal Bacteria
3.1. Flavonoid Quercetin
3.2. Proanthocyanidin
3.3. Anthocyanidins
3.4. Curcumin
3.5. Resveratrol
3.6. Ellagitannin
4. Physiological Functions Mediated by Intestinal Microbiota
4.1. Bioavailability and Action of Polyphenol Catabolites
4.2. Action of Polyphenols on Gut Microbiota
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alkhalidy, H.; Wang, Y.; Liu, D. Dietary flavonoids in the prevention of T2D: An overview. Nutrients 2018, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Sheen, J.M.; Hu, W.L.; Hung, Y.C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, polyphenols and tannins: An overview. In Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 1–24. [Google Scholar] [CrossRef]
- Terao, J. Flavonoids: Metabolism bioavailability, and health impacts. In Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology; Fraga, C.G., Ed.; John Wiley & Sons: Hoboken NJ, USA, 2009; pp. 185–196. [Google Scholar] [CrossRef]
- Bentsáth, A.; Rusznyák, S.; Szent-Györgyi, A. Vitamin nature of flavones [1]. Nature 1936, 138, 798. [Google Scholar] [CrossRef]
- Almeida Rezende, B.; Pereira, A.C.; Cortes, S.F.; Lemos, V.S. Vascular effects of flavonoids. Curr. Med. Chem. 2016, 23, 87–102. [Google Scholar] [CrossRef]
- Hertog, M.G.L.; Feskens, E.J.M.; Kromhout, D.; Hertog, M.G.L.; Hollman, P.C.H.; Hertog, M.G.L.; Katan, M.B. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailablity and bioefficacy of poly-phenols in humans, II: Review of 93 intervention studies. Am.J.Clin.Nutr. 2005, 81, 2435–2555. [Google Scholar] [CrossRef]
- Terao, J. Dietary flavonoids as antioxidants. Forum Nutr. 2009, 61, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The Biological Relevance of Direct Antioxidant Effects of Polyphenols for Cardiovascular Health in Humans Is Not Established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, A.; Ohnishi, K. Target molecules of food phytochemicals: Food science bound for the next dimension. Food Funct. 2012, 3, 462–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef] [Green Version]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut Microbes and the Brain: Paradigm Shift in Neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shi, F.; Guo, Z.; Zhao, J.; Song, X.; Yang, H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol. Carcinog. 2018, 57, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Savi, M.; Bocchi, L.; Bresciani, L.; Falco, A.; Quaini, F.; Mena, P.; Brighenti, F.; Crozier, A.; Stilli, D.; Del Rio, D. Trimethylamine-N-oxide (TMAO)-induced impairment of cardiomyocyte function and the protective role of urolithin B-glucuronide. Molecules 2018, 23, 549. [Google Scholar] [CrossRef]
- Kasimsetty, S.G.; Bialonska, D.; Reddy, M.K.; Thornton, C.; Willett, K.L.; Ferreira, D. Effects of pomegranate chemical constituents/intestinal microbial metabolites on CYP1B1 in 22Rv1 prostate cancer cells. J. Agric. Food Chem. 2009, 57, 10636–10644. [Google Scholar] [CrossRef]
- Miene, C.; Weise, A.; Glei, M. Impact of polyphenol metabolites produced by colonic microbiota on expression of COX-2 and GSTT2 in human colon cells (LT97). Nutr. Cancer 2011, 63, 653–662. [Google Scholar] [CrossRef]
- Surapaneni, K.M.; Priya, V.V.; Mallika, J. Pioglitazone, quercetin and hydroxy citric acid effect on cytochrome P450 2E1 (CYP2E1) enzyme levels in experimentally induced non alcoholic steatohepatitis (NASH). Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2736–2741. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases - Promises, Perspectives, and Pitfalls. Oxid. Med. Cell. Longev. 2016, 9346470. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Van Der Hooft, J.J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols1-3. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef] [PubMed]
- Pforte, H.; Hempel, J.; Jacobasch, G. Distribution pattern of a flavonoid extract in the gastrointestinal lumen and wall of rats. Nahrung 1999, 43, 205–208. [Google Scholar] [CrossRef]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Donovan, J.L.; Lee, A.; Manach, C.; Rios, L.; Morand, C.; Scalbert, A.; Rémésy, C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br. J. Nutr. 2002, 87, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.E.; Chaudry, F.; Pannala, A.S.; Srai, S.K.; Debnam, E.; Rice-Evans, C. Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophys. Res. Commun. 2000, 272, 236–241. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [CrossRef] [Green Version]
- Piskula, M.K.; Murota, K.; Terao, J. Bioavailability of flavonols and flavones. In Flavonoids and Related Compounds- Bioavailability and Function; Spencer, J.P., Crozier, A., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 93–108. [Google Scholar]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- Aura, A.M.; O’Leary, K.A.; Williamson, G.; Ojala, M.; Bailey, M.; Puupponen-Pimiä, R.; Nuutila, A.M.; Oksman-Caldentey, K.M.; Poutanen, K. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J. Agric. Food Chem. 2002, 50, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Natsume, M.; Osakabe, N.; Oyama, M.; Sasaki, M.; Baba, S.; Nakamura, Y.; Osawa, T.; Terao, J. Structures of (-)-epicatechin glucuronide identified from plasma and urine after oral ingestion of (-)-epicatechin: Differences between human and rat. Free Radic. Biol. Med. 2003, 34, 840–849. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Yasuda, A.; Natsume, M.; Takizawa, T.; Nakamura, T.; Terao, J. Bioavailability of (-)-epicatechin upon intake of chocolate and cocoa in human volunteers. Free Radic. Res. 2000, 33, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, J.I.; Borges, G.; Momma, T.Y.; Spencer, J.P.E.; Keen, C.L.; Crozier, A.; Schroeter, H. The metabolome of [2-14C](-)-epicatechin in humans: Implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives. Sci. Rep. 2016, 6, 29034. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.J.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334. [Google Scholar] [CrossRef]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009, 53, 44–53. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Ou, K.; Percival, S.S.; Zou, T.; Khoo, C.; Gu, L. Transport of cranberry A-type procyanidin dimers, trimers, and tetramers across monolayers of human intestinal epithelial caco-2 cells. J. Agric. Food Chem. 2012, 60, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Akiyama, H.; Kanda, T.; Ohtake, Y.; Goda, Y. Apple procyanidin oligomers absorption in rats after oral administration: Analysis of procyanidins in plasma using the porter method and high-performance liquid chromatography/tandem mass spectrometry. J. Agric. Food Chem. 2006, 54, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.; MacI, A.; Romero, M.P.; Valls, J.; Bladé, C.; Arola, L.; Motilva, M.J. Bioavailability of procyanidin dimers and trimers and matrix food effects in in vitro and in vivo models. Br. J. Nutr. 2010, 103, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Terao, J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4β-8)-epicatechin] in rats. Free Radic. Biol. Med. 2002, 33, 142–148. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Gruppen, H.; Hollman, P.C.H. Procyanidin Dimers A1, A2, and B2 Are Absorbed without Conjugation or Methylation from the Small Intestine of Rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [Green Version]
- Barnes, R.C.; Krenek, K.A.; Meibohm, B.; Mertens-Talcott, S.U.; Talcott, S.T. Urinary metabolites from mango (Mangifera indica L. cv. Keitt) galloyl derivatives and in vitro hydrolysis of gallotannins in physiological conditions. Mol. Nutr. Food Res. 2016, 60, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef]
- González-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Serra, A.; MacIà, A.; Romero, M.P.; Reguant, J.; Ortega, N.; Motilva, M.J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem. 2012, 130, 383–393. [Google Scholar] [CrossRef]
- Hein, E.M.; Rose, K.; Van’t Slot, G.; Friedrich, A.W.; Humpf, H.U. Deconjugation and degradation of flavonol glycosides by pig cecal microbiota characterized by fluorescence in situ hybridization (FISH). J. Agric. Food Chem. 2008, 56, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, Z.; Zhang, N.; Liu, L.; Li, S.; Wei, H. In vitro catabolism of quercetin by human fecal bacteria and the antioxidant capacity of its catabolites. Food Nutr. Res. 2014, 58, 23406. [Google Scholar] [CrossRef]
- Schneider, H.; Simmering, R.; Hartmann, L.; Pforte, H.; Blaut, M. Degradation of quercetin-3-glucoside in gnotobiotic rats associated with human intestinal bacteria. J. Appl. Microbiol. 2000, 89, 1027–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadwick, R.W.; George, S.E.; Claxton, L.D. Role of the gastrointestinal mucosa and microflora in the bioactivation of dietary and environmental mutagens or carcinogens. Drug Metab. Rev. 1992, 24, 425–492. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Fujita, Y. Excess production of Bacillus subtilis quercetin 2,3-dioxygenase affects cell viability in the presence of quercetin. Biosci. Biotechnol. Biochem. 2010, 74, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Schaab, M.R.; Barney, B.M.; Francisco, W.A. Kinetic and spectroscopic studies on the quercetin 2,3-dioxygenase from Bacillus subtilis. Biochemistry 2006, 45, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Huang, Q.Q.; Li, P.; Zhang, J.J. Catalytic dioxygenation of flavonol by MII-complexes (M = Mn, Fe, Co, Ni, Cu and Zn)-mimicking the MII-substituted quercetin 2,3-dioxygenase. Dalt. Trans. 2015, 44, 13926–13938. [Google Scholar] [CrossRef] [PubMed]
- Fetzner, S. Ring-Cleaving Dioxygenases with a Cupin Fold. Appl. Environ. Microbiol. 2012, 78, 2505–2514. [Google Scholar] [CrossRef] [Green Version]
- Ulbrich, K.; Reichardt, N.; Braune, A.; Krohd, L.W.; Blaut, M.; Rohn, S. The microbial degradation of onion flavonol glucosides and their roasting products by the human gut bacteria Eubacterium ramulus and Flavonifractor plautii. Food Res. Int. 2015, 67, 349–355. [Google Scholar] [CrossRef]
- Heinken, A.; Thiele, I. Anoxic Conditions Promote Species-Specific Mutualism between Gut Microbes In Silico. Appl Env. Microbiol 2015, 81, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Hypoxia and gastrointestinal disease. J. Mol. Med. 2007, 85, 1295–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, T.; Troup, B.; Szabo, A.; Hungerer, C.; Jahn, D. The anaerobic life of Bacillus subtilis: Cloning of the genes encoding the respiratory nitrate reductase system. FEMS Microbiol. Lett. 1995, 131, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.A.; Khaneja, R.; Tam, N.M.; Cazzato, A.; Tan, S.; Urdaci, M.; Brisson, A.; Gasbarrini, A.; Barnes, I.; Cutting, S.M. Bacillus subtilis isolated from the human gastrointestinal tract. Res. Microbiol. 2009, 160, 134–143. [Google Scholar] [CrossRef]
- Bowater, L.; Fairhurst, S.A.; Just, V.J.; Bornemann, S. Bacillus subtilis YxaG is a novel Fe-containing quercetin 2,3-dioxygenase. FEBS Lett. 2004, 557, 45–48. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, S.E.; Frederiksen, H.; Krogholm, K.S.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Aura, A.M.; Hollman, P.C.H.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef]
- Barroso, E.; Sánchez-Patán, F.; Martín-Alvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; Requena, T.; Van De Wiele, T.; Martínez-Cuesta, M.C. Lactobacillus plantarum IFPL935 favors the initial metabolism of red wine polyphenols when added to a colonic microbiota. J. Agric. Food Chem. 2013, 61, 10163–10172. [Google Scholar] [CrossRef]
- Goodrich, K.M.; Neilson, A.P. Simultaneous UPLC-MS/MS analysis of native catechins and procyanidins and their microbial metabolites in intestinal contents and tissues of male Wistar Furth inbred rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 958, 63–74. [Google Scholar] [CrossRef]
- Jiménez-Girón, A.; Ibáñez, C.; Cifuentes, A.; Simó, C.; Muñoz-González, I.; Martín-Álvarez, P.J.; Bartolomé, B.; Victoria Moreno-Arribas, V. Faecal metabolomic fingerprint after moderate consumption of red wine by healthy subjects. J. Proteome Res. 2015, 14, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Pons, Z.; Muguerza, B.; Arola-Arnal, A. A rapid method to determine colonic microbial metabolites derived from grape flavanols in rat plasma by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 7698–7706. [Google Scholar] [CrossRef] [PubMed]
- Mülek, M.; Högger, P. Highly sensitive analysis of polyphenols and their metabolites in human blood cells using dispersive SPE extraction and LC-MS/MS. Anal. Bioanal. Chem. 2015, 407, 1885–1899. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Catabolism of (+)-catechin and (-)-epicatechin by rat intestinal microbiota. J. Agric. Food Chem. 2013, 61, 4927–4935. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, I.; Janssen, M.; Netzel, M.; Strass, G.; Frank, T. Bioavailability of anthocyanidin-3-glycosides following consumption of elderberry extract and blackcurrant juice. Int. J. Clin. Pharmacol. Ther. 2004, 42, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A13C-tracer study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Felgines, C.; Verine, S.; Ra, T.; Gonthier, M.-P.; Texier, O.; Scalbert, A.; Lamaison, J.-L.; Ré Mé Sy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. J. Nutr. 2003, 133, 1296–1301. [Google Scholar] [CrossRef]
- Mertens-Talcott, S.U.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.A.; Meibohm, B.; Talcott, S.T.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich açai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Shida, Y.; Rahman, M.M.; Sekiya, M.; Hatano, Y.; Matsumoto, H.; Hirayama, M.; Konishi, T.; Ikeshiro, Y. Effect on both aglycone and sugar moiety towards Phase II metabolism of anthocyanins. Food Chem. 2008, 110, 493–500. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G.; Holub, B.J.; Wang, J. Anthocyanin metabolites in human urine and serum. Br. J. Nutr. 2004, 91, 933–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.B. Organic Chemistry of Enzyme-Catalyzed Reactions; Revised ed.; Academic Press: London, UK, 2002; pp. 227–250. ISBN 9780080513362. [Google Scholar]
- Kang, S.; Joo, C.; Kim, S.M.; Han, H.; Yang, J.W. Oxidation of benzoins to benzoic acids using sodium hydride under oxygen atmosphere. Tetrahedron Lett. 2011, 52, 502–504. [Google Scholar] [CrossRef]
- Amin, H.P.; Czank, C.; Raheem, S.; Zhang, Q.; Botting, N.P.; Cassidy, A.; Kay, C.D. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol. Nutr. Food Res. 2015, 59, 1095–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Woodward, G.M.; Needs, P.W.; Kay, C.D. Anthocyanin-derived phenolic acids form glucuronides following simulated gastrointestinal digestion and microsomal glucuronidation. Mol. Nutr. Food Res. 2011, 55, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the Cancer Chemopreventive Agent Curcumin in Human and Rat Intestine. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 105–111. [Google Scholar] [PubMed]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- González, C.A.; Pera, G.; Agudo, A.; Bueno-De-Mesquita, H.B.; Ceroti, M.; Boeing, H.; Schulz, M.; Del Giudice, G.; Plebani, M.; Carneiro, F.; et al. Fruit and vegetable intake and the risk of stomach and oesophagus adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Int. J. Cancer 2006, 118, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate Juice Ellagitannin Metabolites Are Present in Human Plasma and Some Persist in Urine for Up to 48 Hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Álvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A.; et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Sánchez, M.A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; Pastor-Quirante, F.A.; et al. Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Larrosa, M.; García-Conesa, M.T.; Cerdá, B.; Vidal-Guevara, M.L.; Tomás-Barberán, F.A.; Espín, J.C. Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J. Agric. Food Chem. 2012, 60, 5749–5754. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Urpi-Sarda, M.; García-Villalba, R.; Rabassa, M.; López-Uriarte, P.; Bulló, M.; Jáuregui, O.; Tomás-Barberán, F.; Salas-Salvadó, J.; Espín, J.C.; et al. Urolithins are the main urinary microbial-derived phenolic metabolites discriminating a moderate consumption of nuts in free-living subjects with diagnosed metabolic syndrome. J. Agric. Food Chem. 2012, 60, 8930–8940. [Google Scholar] [CrossRef]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2009, 42, 74–83. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic Acid Is the Major Human Metabolite of Cyanidin-Glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKay, D.L.; Chen, C.Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.; Betalleluz-Pallardel, I.; Chirinos, R.; Aguilar-Galvez, A.; Noratto, G.; Pedreschi, R. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012, 135, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, N.; Matsumoto, M.; Chiji, H.; Hara, H. Oligosaccharide promotes bioavailability of a water-soluble flavonoid glycoside, αG-rutin, in rats. J. Agric. Food Chem. 2009, 57, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Zhang, Y.; McKeever, R.; Henning, S.M.; Lee, R.; Suchard, M.A.; Li, Z.; Chen, S.; Thames, G.; Zerlin, A.; et al. Pomegranate Juice and Extracts Provide Similar Levels of Plasma and Urinary Ellagitannin Metabolites in Human Subjects. J. Med. Food 2008, 11, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Daniel, E.M.; Ratnayake, S.; Kinstle, T.; Stoner, G.D. The effects of pH and rat intestinal contents on the liberation of ellagic acid from purified and crude ellagitannins. J. Nat. Prod. 1991, 54, 946–952. [Google Scholar] [CrossRef]
- Dueñas, M.; Surco-Laos, F.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Antioxidant properties of major metabolites of quercetin. Eur. Food Res. Technol. 2011, 232, 103–111. [Google Scholar] [CrossRef]
- Has, A.; Activity, A.; Gao, K.; Xu, A.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; et al. Of the Major Phenolic Acids Formed during Human Microbial Fermentation of Tea, Citrus, and Soy Flavonoid Supplements, Only. Joournal Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef]
- Tang, J.S.; Vissers, M.C.M.; Anderson, R.F.; Sreebhavan, S.; Bozonet, S.M.; Scheepens, A.; Melton, L.D. Bioavailable Blueberry-Derived Phenolic Acids at Physiological Concentrations Enhance Nrf2-Regulated Antioxidant Responses in Human Vascular Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1700647. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugiyama, Y.; Sakano, T.; Ohigashi, H. Flavonols enhanced production of anti-inflammatory substance(s) by Bifidobacterium adolescentis: Prebiotic actions of galangin, quercetin, and fisetin. BioFactors 2013, 39, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, X.L.; Li, T.Y.; Li, M.Y.; Huang, R.M.; Li, W.; Yang, R.L. 3-(4-Hydroxyphenyl)propionic acid, a major microbial metabolite of procyanidin A2, shows similar suppression of macrophage foam cell formation as its parent molecule. RSC Adv. 2018, 8, 6242–6250. [Google Scholar] [CrossRef] [Green Version]
- Dobroslawa, B.; Kasimsetty, S.G.; Khan, S.I.; Daneel, F. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Larrosa, M.; Toms-Barberán, F.A.; Dolara, P.; Espín, J.C. NF-κB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br. J. Nutr. 2010, 104, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microbiol. 2005, 98, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory role of grape pomace polyphenols on Lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Zamora-Gasga, V.M.; Venema, K. Prebiotic effect of predigested mango peel on gut microbiota assessed in a dynamic in vitro model of the human colon (TIM-2). Food Res. Int. 2017, in press. [Google Scholar] [CrossRef]
- Cueva, C.; Moreno-Arribas, M.V.; Martín-Alvarez, P.J.; Bills, G.; Vicente, M.F.; Basilio, A.; Rivas, C.L.; Requena, T.; Rodríguez, J.M.; Bartolomé, B. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res. Microbiol. 2010, 161, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; González-Sarrías, A.; Toti, S.; Cerón, J.J.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Mcfadden, R.M.T.; Larmonier, C.B.; Shehab, K.W.; Midura-Kiela, M.; Ramalingam, R.; Harrison, C.A.; Besselsen, D.G.; Chase, J.H.; Caporaso, J.G.; Jobin, C.; et al. The role of curcumin in modulating colonic microbiota during colitis and colon cancer prevention. Inflamm. Bowel Dis. 2015, 21, 2483–2494. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol Attenuates Trimethylamine-N-Oxide (TMAO)-Induced Atherosclerosis by Regulating TMAO Synthesis and Bile Acid Metabolism via Remodeling of the Gut Microbiota. MBio 2016, 7, e02210-15. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Ho, C.T.; Guo, X.; Wu, Z.; Weng, P.; Yan, M.; Cao, J. Metagenomics analysis of gut microbiota modulatory effect of green tea polyphenols by high fat diet-induced obesity mice model. J. Funct. Foods 2018, 46, 268–277. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia municiphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- De La Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care 2017, 40, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Bustos, I.; García-Cayuela, T.; Hernández-Ledesma, B.; Peláez, C.; Requena, T.; Martínez-Cuesta, M.C. Effect of flavan-3-ols on the adhesion of potential probiotic lactobacilli to intestinal cells. J. Agric. Food Chem. 2012, 60, 9082–9088. [Google Scholar] [CrossRef]
- Volstatova, T.; Marsik, P.; Rada, V.; Geigerova, M.; Havlik, J. Effect of apple extracts and selective polyphenols on the adhesion of potential probiotic strains of Lactobacillus gasseri R and Lactobacillus casei FMP. J. Funct. Foods 2017, 35, 391–397. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Zduńczyk, Z.; Juśkiewicz, J.; Estrella, I. Cecal parameters of rats fed diets containing grapefruit polyphenols and inulin as single supplements or in a combination. Nutrition 2006, 22, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Rémésy, C.; Demigné, C. Apple Pectin and a Polyphenol-Rich Apple Concentrate Are More Effective Together Than Separately on Cecal Fermentations and Plasma Lipids in Rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Milala, J.; Jurgoński, A.; Karlińska, E.; Zduńczyk, Z.; Juśkiewicz, J. Strawberry ellagitannins thwarted the positive effects of dietary fructooligosaccharides in rat cecum. J. Agric. Food Chem. 2014, 62, 5871–5880. [Google Scholar] [CrossRef] [PubMed]
- Fotschki, B.; Juśkiewicz, J.; Jurgoński, A.; Kołodziejczyk, K.; Milala, J.; Kosmala, M.; Zduńczyk, Z. Anthocyanins in strawberry polyphenolic extract enhance the beneficial effects of diets with fructooligosaccharides in the rat cecal environment. PLoS ONE 2016, 11, e0149081. [Google Scholar] [CrossRef]
- Kawabata, K.; Kato, Y.; Sakano, T.; Baba, N.; Hagiwara, K.; Tamura, A.; Baba, S.; Natsume, M.; Ohigashi, H. Effects of phytochemicals on in vitro anti-inflammatory activity of Bifidobacterium adolescentis. Biosci. Biotechnol. Biochem. 2015, 79, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Ménard, S.; Candalh, C.; Bambou, J.C.; Terpend, K.; Cerf-Bensussan, N.; Heyman, M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut 2004, 53, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokhlova, E.V.; Smeianov, V.V.; Efimov, B.A.; Kafarskaia, L.I.; Pavlova, S.I.; Shkoporov, A.N. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol. Immunol. 2012, 56, 27–39. [Google Scholar] [CrossRef]
- Imaoka, A.; Shima, T.; Kato, K.; Mizuno, S.; Uehara, T.; Matsumoto, S.; Setoyana, H.; Hara, T.; Umesaki, Y. Anti-inflammatory activity of probiotic Bifidobacterium: Enhancement of IL-10 production in peripheral blood mononuclear cells from ulcerative colitis patients and inhibition of IL-8 secretion in HT-29 cells. World J. Gastroenterol. 2008, 14, 2511–2516. [Google Scholar] [CrossRef]
- Kawabata, K.; Baba, N.; Sakano, T.; Hamano, Y.; Taira, S.; Tamura, A.; Baba, S.; Natsume, M.; Ishii, T.; Murakami, S.; et al. Functional properties of anti-inflammatory substances from quercetin-treated Bifidobacterium adolescentis. Biosci. Biotechnol. Biochem. 2018, 82, 689–697. [Google Scholar] [CrossRef]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Kunisawa, J. Gut microbiome, metabolome, and allergic diseases. Allergol. Int. 2017, 66, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Kunisawa, J.; Kiyono, H. Dietary and microbial metabolites in the regulation of host immunity. Front. Microbiol. 2017, 8, 2171. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Samples | Subjects (Gender; Age; BMI) | Detected Compounds | Cmax1 (M) | Tmax1 (hours) | Cmax2 (M) | Tmax2 (hours) | AUC0-last (M·h) | T1/2 (h) | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Blood orange juice (71 mg cyanidin glucosides) | Healthy men & women; 20–24; 21–31 | Cyanidin-3-glucoside | 0.0019 | 0.5 | 0.007 ac | 116 | |||
| 3,4-Dihydroxybenzoic acid | 0.492 | 2 | 11 ac | ||||||
| Cranberry juice cocktail (188.5 mg phenolics) | Healthy nonsmoking men & postmenopausal women; 50–70; 18.5–29.9 | Quercetin | 1.1 | 1.4 | 1.5 | 7.8 | 9.9 bc | 117 | |
| Epicatechin | 0.082 | 2.6 | 0.079 | 8.1 | 0.586 bc | ||||
| Myricetin | 0.068 | 1.7 | 0.05 | 7.8 | 0.319 bc | ||||
| Isorhamnetin | 0.099 | 0.5 | 0.153 | 7.3 | 0.267 bc | ||||
| Peonidin glycosides | 0.00017–0.0021 | 0.9–4.7 | 0.00038–0.0053 bc | ||||||
| Cyanidin glycosides | 0.00019–0.00073 | 1.7–3.3 | 0.000092–0.0033 bc | ||||||
| 3,4-Dihydroxybenzoic acid | 10 | 8.8 | 91 bc | ||||||
| 4-Hydroxy-3-methoxybenzoic acid | 12 | 0.7 | 11 | 6.1 | 16 bc | ||||
| 4-Hydroxybenzoic acid | 1.4 | 0.8 | 1.4 | 7.2 | 9.9 bc | ||||
| 4-Hydroxyphenylacetic acid | 0.613 | 1.5 | 0.783 | 7.8 | 2.3 bc | ||||
| 3,4-Dihydroxyphenylacetic acid | 0.083 | 8.4 | 0.348 bc | ||||||
| [2-14C](−)-Epicatechin (207 mmoles) | Healthy men; 31 ± 3; 24.5 ± 3.3 | Epicatechin conjugates g | 1.223 | 1 | 4.943 b | 1.9 | 50 | ||
| 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone | 0.272 d | 6.4 | 7.595 bd | 6.3 | |||||
| 0.177 e | 6.1 | 3.237 be | 4.4 | ||||||
| 0.039 f | 5.5 | 1.017 bf | 6.4 | ||||||
| 5-(3-Hydroxyphenyl)-4-hydroxyvaleric acid | 0.056 d | 5.9 | 1.492 bd | 7.6 | |||||
| 5-(3,4-Dihydroxyphenyl)-4-hydroxyvaleric acid | 0.054 e | 4.9 | 0.835 be | 6.5 | |||||
| Pomegranate juice (318 mg of punicalagin; 12 mg of ellagic acid) | Healthy men & women; 32.6 ± 10.2; 21.3 ± 1.4 | Ellagic acid | 0.06 | 0.98 | 0.17 a | 0.71 | 107 | ||
| Pomegranate juice (857 mg of gallic acid equivalent) | Healthy men & women; 29.7 ± 8.3; 24.1 ± 3.6 | Ellagic acid | 0.06 | 0.65 | 0.14 a | 1.14 | 120 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24020370
Kawabata K, Yoshioka Y, Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019; 24(2):370. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24020370
Chicago/Turabian StyleKawabata, Kyuichi, Yasukiyo Yoshioka, and Junji Terao. 2019. "Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols" Molecules 24, no. 2: 370. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24020370